The Food and Drug Administration (FDA) has conditionally approved the antiviral drug remdesivir to treat people with severe COVID-19, the Central Epidemic Command Center (CECC) said yesterday.
At the CECC’s daily news conference in Taipei, Minister of Health and Welfare Chen Shih-chung (陳時中), who heads the center, said that no new cases have been confirmed, while 421 people have been released from isolation after contracting the novel coronavirus.
“Taiwan remains the same — with no new domestic cases reported in 48 consecutive days,” Chen said. “However, the COVID-19 situation globally is still serious and almost 6 million cases have been confirmed, so we have to be extra careful about border controls.”

Photo: CNA
After a specialist meeting on Friday, the FDA approved remdesivir to treat people with severe COVID-19, Chen said.
FDA Director-General Wu Shou-mei (吳秀梅) said that to respond to the pandemic, Taiwan needs to prepare treatments, so the administration reached out to pharmaceutical companies.
Gilead Sciences Inc, which produces the experimental antiviral drug under the brand name Veklury, on Monday submitted documents to have the drug approved, and the FDA and the Center for Drug Evaluation immediately launched a review, Wu said.

Photo: CNA
The review was completed by Thursday and with the meeting on Friday, the agencies decided that based on Article 48 of the Pharmaceutical Affairs Act (藥事法), conditional approval would be granted to import Veklury, she said.
The drug can be used to treat severe cases in adults and children, with treatment limited to a maximum of 10 days, she said.
Gilead must enforce a risk management plan and the results of new clinical trials must be immediately sent to the FDA for review, while technical data must be collated within a year, Wu said.
Adult patients are to have 200mg of the drug injected on the first day and receive a 100mg injection once per day from the second day, while children are to be given the same doses if they weigh at least 40kg, she said, adding that dosages would be adjusted according to body weight for children who weigh less than 40kg.
“As many clinical trials are still ongoing, it [remdesivir] is so far one of the most promising drugs we have observed, but the drug approval was granted quickly in response to the emergency need in Taiwan,” Wu said.
The US Food and Drug Administration on May 1 gave the drug emergency use authorization for people hospitalized with severe COVID-19, while Japan on May 7 was the first nation to grant it regulatory approval.
Taiwan is the second nation in the world to have approved the drug.
CECC advisory specialist panel convener Chang Shan-chwen (張上淳) said that although evidence might not yet be sufficient, information on the drug’s effects has been positive, so most specialists support importing the drug to prepare the health system for the possibility of another outbreak.
The drug would only be administered with the informed consent of the patient, and their kidney and liver functions would be monitored to check for adverse reactions, Chang said.
Remdesivir was developed to treat Ebola, but clinical trials showed that it was not effective enough, Wu said.
However, it improved symptoms in severe COVID-19 cases in clinical trials, in which 11 Taiwanese participated, she said.
After the conditional approval is officially granted next week, the FDA plans to order 1,000 doses, which are expected to arrive in Taiwan by late July at the earliest, she said.

NATIONAL SECURITY THREAT: An official said that Guan Guan’s comments had gone beyond the threshold of free speech, as she advocated for the destruction of the ROC China-born media influencer Guan Guan’s (關關) residency permit has been revoked for repeatedly posting pro-China content that threatens national security, the National Immigration Agency said yesterday. Guan Guan has said many controversial things in her videos posted to Douyin (抖音), including “the red flag will soon be painted all over Taiwan” and “Taiwan is an inseparable part of China,” while expressing hope for expedited “reunification.” The agency received multiple reports alleging that Guan Guan had advocated for armed reunification last year. After investigating, the agency last month issued a notice requiring her to appear and account for her actions. Guan Guan appeared as required,
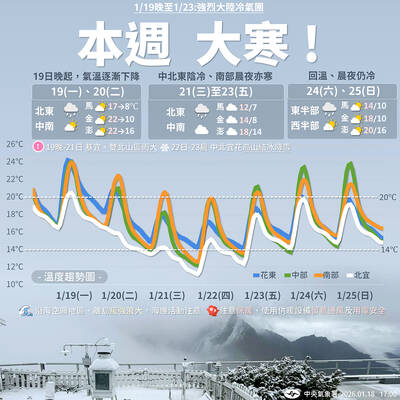
A strong cold air mass is expected to arrive tonight, bringing a change in weather and a drop in temperature, the Central Weather Administration (CWA) said. The coldest time would be early on Thursday morning, with temperatures in some areas dipping as low as 8°C, it said. Daytime highs yesterday were 22°C to 24°C in northern and eastern Taiwan, and about 25°C to 28°C in the central and southern regions, it said. However, nighttime lows would dip to about 15°C to 16°C in central and northern Taiwan as well as the northeast, and 17°C to 19°C elsewhere, it said. Tropical Storm Nokaen, currently

‘NATO-PLUS’: ‘Our strategic partners in the Indo-Pacific are facing increasing aggression by the Chinese Communist Party,’ US Representative Rob Wittman said The US House of Representatives on Monday released its version of the Consolidated Appropriations Act, which includes US$1.15 billion to support security cooperation with Taiwan. The omnibus act, covering US$1.2 trillion of spending, allocates US$1 billion for the Taiwan Security Cooperation Initiative, as well as US$150 million for the replacement of defense articles and reimbursement of defense services provided to Taiwan. The fund allocations were based on the US National Defense Authorization Act for fiscal 2026 that was passed by the US Congress last month and authorized up to US$1 billion to the US Defense Security Cooperation Agency in support of the

PAPERS, PLEASE: The gang exploited the high value of the passports, selling them at inflated prices to Chinese buyers, who would treat them as ‘invisibility cloaks’ The Yilan District Court has handed four members of a syndicate prison terms ranging from one year and two months to two years and two months for their involvement in a scheme to purchase Taiwanese passports and resell them abroad at a massive markup. A Chinese human smuggling syndicate purchased Taiwanese passports through local criminal networks, exploiting the passports’ visa-free travel privileges to turn a profit of more than 20 times the original price, the court said. Such criminal organizations enable people to impersonate Taiwanese when entering and exiting Taiwan and other countries, undermining social order and the credibility of the nation’s