Test results on noodles produced by South Korea’s Nongshim Co that were reported to contain traces of benzopyrene and were recalled in South Korea last month, showed low levels of benzopyrene that were within the limits set under international standards, the Department of Health said yesterday.
The Food and Drug Administration (FDA) said shortly after South Korea recalled six Nongshim products, including two types found to contain a known carcinogen, benzopyrene, the agency immediately ordered two Nongshim-brand noodles sold in Taiwan — Neoguri seafood mild noodles and Neoguri seafood spicy noodles — to be taken off store shelves on Oct. 25.
In addition, the FDA said it had asked the New Taipei City (新北市) Government’s health department to inspect all imported Nongshim products and to send samples to the agency for laboratory testing.
Among five samples tested, the spice powders of two Neoguri seafood mild noodles samples were found to contain benzopyrene levels of 1.01 parts per billion (ppb) and 0.97ppb, the FDA said.
The levels of benzopyrene found in the tests were below the limits stipulated by the EU and the WHO, it said, adding that with a benzopyrene concentration of 1.01ppb, a person who weighs 60kg would ingest about 0.012mcg of benzopyrene from one bag of instant noodles, lower than the estimated average daily intake of 0.24mcg (for a person of 60kg) calculated by the Joint Expert Committee on Food Additives.
Because the amount of spice powder eaten with instant noodles is small, the exposure to benzopyrene is not considered harmful to human health, the FDA added.
At the Legislative Yuan yesterday, FDA Director-General Kang Jaw-jou (康照洲) said: “We will quickly do a background survey on all food products, and set a standard for the substance after we have gained enough information and have discussed it with specialists.”

A preclearance service to facilitate entry for people traveling to select airports in Japan would be available from Thursday next week to Feb. 25 at Taiwan Taoyuan International Airport, Taoyuan International Airport Corp (TIAC) said on Tuesday. The service was first made available to Taiwanese travelers throughout the winter vacation of 2024 and during the Lunar New Year holiday. In addition to flights to the Japanese cities of Hakodate, Asahikawa, Akita, Sendai, Niigata, Okayama, Takamatsu, Kumamoto and Kagoshima, the service would be available to travelers to Kobe and Oita. The service can be accessed by passengers of 15 flight routes operated by
Chinese spouse and influencer Guan Guan’s (關關) residency permit has been revoked for repeatedly posting pro-China videos that threaten national security, the National Immigration Agency confirmed today. Guan Guan has said many controversial statements in her videos posted to Douyin (抖音), including “the red flag will soon be painted all over Taiwan” and “Taiwan is an inseparable part of China,” and expressing hope for expedited reunification. The agency last year received multiple reports alleging that Guan Guan had advocated for armed reunification. After verifying the reports, the agency last month issued a notice requiring her to appear and explain her actions. Guan

GIVE AND TAKE: Blood demand continues to rise each year, while fewer young donors are available due to the nation’s falling birthrate, a doctor said Blood donors can redeem points earned from donations to obtain limited edition Formosan black bear travel mugs, the Kaohsiung Blood Center said yesterday, as it announced a goal of stocking 20,000 units of blood prior to the Lunar New Year. The last month of the lunar year is National Blood Donation Month, when local centers seek to stockpile blood for use during the Lunar New Year holiday. The blood demand in southern Taiwan — including Tainan and Kaohsiung, as well as Chiayi, Pingtung, Penghu and Taitung counties — is about 2,000 units per day, the center said. The donation campaign aims to boost
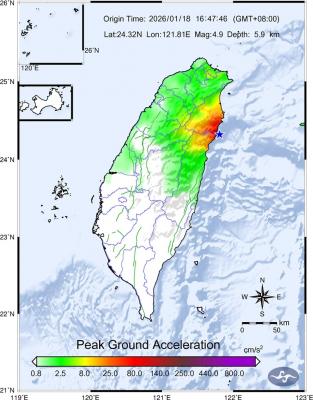
The Central Weather Administration (CWA) said a magnitude 4.9 earthquake that struck off the coast of eastern Taiwan yesterday was an independent event and part of a stress-adjustment process. The earthquake occurred at 4:47pm, with its epicenter at sea about 45.4km south of Yilan County Hall at a depth of 5.9km, the CWA said. The quake's intensity, which gauges the actual effects of a temblor, was highest in several townships in Yilan and neighboring Hualien County, where it measured 4 on Taiwan's seven-tier intensity scale, the CWA said. Lin Po-yu (林柏佑), a division chief at the CWA's Seismological Center, told a news conference