Pfizer Inc agreed on Wednesday to plead guilty to a US criminal charge relating to promotion of its now-withdrawn Bextra pain medicine and will pay a record US$2.3 billion to settle allegations it improperly marketed 13 medicines.
The world’s biggest drugmaker was slapped with the huge fines by the US government after being deemed a repeat offender in pitching drugs to patients and doctors for unapproved uses.
Pfizer pleaded guilty in 2004 to an earlier criminal charge of improper sales tactics and its practices have been under US supervision since then.
PHOTO: BLOOMBERG
“If another one of these charges crops up, it would raise questions whether Jeff Kindler is keeping everyone at Pfizer on a tight enough leash,” said Miller Tabak analyst Les Funtleyder, referring to Pfizer’s chief executive officer.
Kindler had been Pfizer’s general counsel from 2002 until taking the helm in 2006. Pfizer declined to comment when asked if he had negotiated the 2004 settlement, and whether he had recommended any safeguards at the time to prevent the kind of recurrent improprieties described on Wednesday.
The company in January said it took a US$2.3 billion charge late last year to resolve allegations involving Bextra and other drugs, but did not provide details at the time.
“The size and seriousness of this resolution, including the huge criminal fine of US$1.3 billion, reflect the seriousness and scope of Pfizer’s crimes,” said Mike Loucks, acting US attorney for the District of Massachusetts.
The settlement includes a US$1.3 billion criminal fine related to methods of selling Bextra, which was withdrawn from the market in 2005 on safety concerns. Pfizer acquired Bextra in its 2003 purchase of Pharmacia Corp.
Pfizer’s marketing team promoted Bextra for acute pain, surgical pain and other unapproved uses, while its sales force promoted the drug directly to doctors for those unapproved uses and dosages, The US Department of Justice said.
The company and Pharmacia also used advisory boards, consultant meetings and provided travel to lavish resorts to improperly promote Bextra to doctors and made misleading claims about the drug’s safety and efficacy, the government said.
The settlement also includes US$1 billion in civil payments related to so-called “off-label” sales of drugs — meaning for uses not authorized by the US Food and Drug Administration — and payments to healthcare professionals. Pfizer denied all of the civil allegations, except for acknowledging improper promotions of the antibiotic Zyvox.
“We regret certain actions taken in the past, but are proud of the action we’ve taken to strengthen our internal controls,” said Amy Schulman, Pfizer’s general counsel.
Justice Department officials said cracking down on fraud in the healthcare industry was a key priority and comes as US President Barack Obama is trying to push through reforms of the US$2.5 trillion healthcare system to clip soaring costs.
The settlement and guilty plea are not expected to significantly hurt Pfizer’s ability to sell drugs, Morningstar analyst Damien Conover said.
Sandra Jordan, a former federal prosecutor and professor at the Charlotte School of Law in North Carolina, said: “Pfizer can survive this and pay the money. If it had fought the government at trial and lost, and a judge imposed a criminal sentence, that could have resulted in a corporate death penalty. That would have put Pfizer out of business.”
The settlement is the largest to date for improper marketing of prescription drugs, topping the US$1.42 billion Eli Lilly and Co agreed to pay earlier this year for off-label sales of its Zyprexa schizophrenia drug.
Pfizer said it will pay US$503 million to resolve practices involving Bextra, US$301 million related to its schizophrenia drug Geodon, US$98 million for Zyvox and about US$50 million for Lyrica, used to treat nerve pain and seizures.

LIMITS: While China increases military pressure on Taiwan and expands its use of cognitive warfare, it is unwilling to target tech supply chains, the report said US and Taiwan military officials have warned that the Chinese People’s Liberation Army (PLA) could implement a blockade within “a matter of hours” and need only “minimal conversion time” prior to an attack on Taiwan, a report released on Tuesday by the US Senate’s China Economic and Security Review Commission said. “While there is no indication that China is planning an imminent attack, the United States and its allies and partners can no longer assume that a Taiwan contingency is a distant possibility for which they would have ample time to prepare,” it said. The commission made the comments in its annual
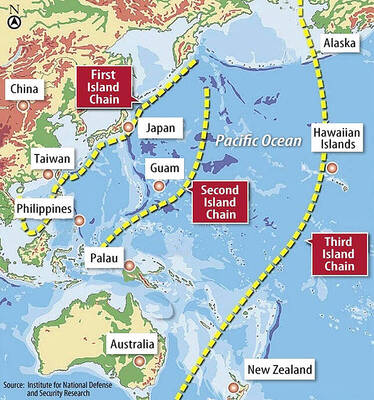
DETERMINATION: Beijing’s actions toward Tokyo have drawn international attention, but would likely bolster regional coordination and defense networks, the report said Japanese Prime Minister Sanae Takaichi’s administration is likely to prioritize security reforms and deterrence in the face of recent “hybrid” threats from China, the National Security Bureau (NSB) said. The bureau made the assessment in a written report to the Legislative Yuan ahead of an oral report and questions-and-answers session at the legislature’s Foreign Affairs and National Defense Committee tomorrow. The key points of Japan’s security reforms would be to reinforce security cooperation with the US, including enhancing defense deployment in the first island chain, pushing forward the integrated command and operations of the Japan Self-Defense Forces and US Forces Japan, as

IN THE NATIONAL INTEREST: Deputy Minister of Foreign Affairs Francois Wu said the strengthening of military facilities would help to maintain security in the Taiwan Strait Japanese Minister of Defense Shinjiro Koizumi, visiting a military base close to Taiwan, said plans to deploy missiles to the post would move forward as tensions smolder between Tokyo and Beijing. “The deployment can help lower the chance of an armed attack on our country,” Koizumi told reporters on Sunday as he wrapped up his first trip to the base on the southern Japanese island of Yonaguni. “The view that it will heighten regional tensions is not accurate.” Former Japanese minister of defense Gen Nakatani in January said that Tokyo wanted to base Type 03 Chu-SAM missiles on Yonaguni, but little progress

NO CHANGES: A Japanese spokesperson said that Tokyo remains consistent and open for dialogue, while Beijing has canceled diplomatic engagements A Japanese official blasted China’s claims that Japanese Prime Minister Sanae Takaichi has altered Japan’s position on a Taiwan crisis as “entirely baseless,” calling for more dialogue to stop ties between Asia’s top economies from spiraling. China vowed to take resolute self-defense against Japan if it “dared to intervene militarily in the Taiwan Strait” in a letter delivered Friday to the UN. “I’m aware of this letter,” said Maki Kobayashi, a senior Japanese government spokeswoman. “The claim our country has altered its position is entirely baseless,” she said on the sidelines of the G20 summit in Johannesburg on Saturday. The Chinese Ministry