Another experimental Alzheimer’s drug can modestly slow patients’ inevitable worsening — by about four to seven months, researchers reported Monday last week.
Eli Lilly and Co. is seeking Food and Drug Administration (FDA) approval of donanemab. If cleared, it would be only the second Alzheimer’s treatment convincingly shown to delay the mind-robbing disease — after the recently approved Leqembi from Japanese drugmaker Eisai.
Lilly announced in May that donanemab appeared to work, but on Monday last week the full results of a study of 1,700 patients was published by the Journal of the American Medical Association (JAMA) and presented at the Alzheimer’s conference.
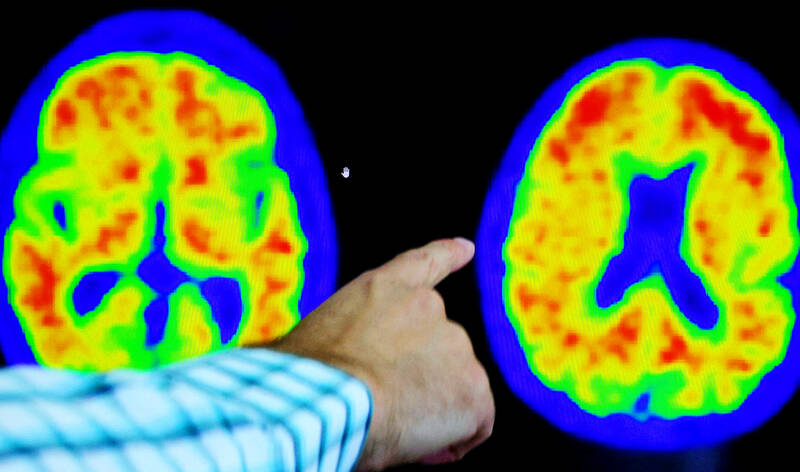
Photo: Reuters 照片:路透
Both donanemab and Leqembi are lab-made antibodies, administered intravenously, that target one Alzheimer’s culprit, sticky amyloid buildup in the brain. And both drugs come with a serious safety concern — brain swelling or bleeding that in the Lilly study was linked to three deaths.
Scientists say while these drugs may mark a new era in Alzheimer’s therapy, huge questions remain about which patients should try them and how much benefit they’ll really notice.
Lilly’s study enrolled people ages 60 to 85 who were in early stages of Alzheimer’s. Half received once-a-month infusions of donanemab and half dummy infusions for 18 months.
Photo: Reuters 照片:路透
The study had a few twists. Patients were switched to dummy infusions if enough amyloid cleared out — something that happened to about half within a year. And because amyloid alone doesn’t cause Alzheimer’s, researchers also tracked levels of another culprit in the brain — abnormal tau protein. More tau signals more advanced disease.
The results: Both groups declined during the 18-month study but overall those given donanemab worsened about 22 percent more slowly. Some patients fared better — those with low to medium tau levels saw a 35 percent slower decline, reflecting that the drug appears to work better in earlier stages of the disease.
How much difference does that make? It means donanemab slowed patients’ worsening by about four to seven months, the JAMA report concluded.
The main safety concern is brain swelling or bleeding, which often causes no symptoms but sometimes can be serious, even fatal. About a quarter of donanemab recipients showed evidence of that swelling, and about 20 percent had microbleeds.
Scientists already know that patients getting any amyloid-targeted therapy need repeat brain scans to check for those side effects — a costly and time-consuming hurdle.
Scientists have long tried and failed to slow Alzheimer’s with amyloid-targeting drugs — and the FDA’s contentious 2021 conditional approval of a drug named Aduhelm soon fizzled amid lack of evidence that it really worked. The approval of Leqembi and promising data for donanemab have reignited interest in attacking amyloid buildup.
(AP)
研究人員上週一發表報告指出,一種試驗中的阿茲海默症藥物,能讓無可避免將惡化的病情減緩約4至7個月。
製藥公司禮來(Eli Lilly)正向美國食品藥物管理局(FDA)申請核准此藥物「多納單抗」(donanemab)。如果過關,將是繼日本衛采製藥(Eisai)最近獲核准的「侖卡奈單抗」(Leqembi)之後,第二種證明有效的療法,可延緩阿茲海默症這種讓腦部喪失功能的疾病。
禮來公司於5月宣布,donanemab似乎有效,對1,700名患者研究的完整結果上週一刊登於《美國醫學會雜誌》(JAMA),並在阿茲海默症會議中發表。
donanemab和Leqembi皆為實驗室製造的抗體,透過靜脈注射給藥,以打擊阿茲海默症的元兇——大腦中粘性類澱粉蛋白的堆積。這兩種藥物都有嚴重的安全疑慮——腦腫脹或出血,禮來公司的研究中有3人的死亡與此相關。
科學家表示,雖然這些藥可能標誌了阿茲海默症治療的新時代,但對於哪些患者應使用這些藥物,以及患者能真正察覺到多少助益,仍有很大的問題。
禮來公司的研究招募了年齡在60歲至85歲之間、處於阿茲海默症早期階段的受試者。一半受試者接受每月一次的donanemab注射,另一半則注射安慰劑,持續18個月。
這項研究有一些轉折。如果被清除的類澱粉蛋白夠多,患者就會改注射安慰劑——這種情況在一年內大約有半數發生。由於類澱粉蛋白本身並不會導致阿茲海默症,因此研究人員還追蹤了大腦中另一個罪魁禍首——異常tau蛋白(或譯「濤蛋白」)的量。tau蛋白的量越多,表示病況越嚴重。
結果:在18個月的研究中,兩組患者的病情均有減輕,但總體而言,接受donanemab治療的患者,病情惡化速度的減緩又更慢了約22%。有些患者的情況較好——tau蛋白水平低至中度的患者,其減緩速度較慢35%,這表示此藥在疾病的早期階段似乎效果更好。
此藥成效如何?這篇登在JAMA的報告得出結論說,這表示donanemab將患者病情的惡化速度減緩了約4到7個月。
主要的安全疑慮是腦腫脹或出血,這通常不會引發任何症狀,但有時可能會很嚴重,甚至致命。大約四分之一的donanemab用藥者出現腦腫脹的跡象,約20%的人有微出血。
科學家已知道,接受任何類澱粉蛋白標靶治療的患者,都需要反覆進行腦部掃描來檢查是否有這些副作用——這是個昂貴且耗時的障礙。
長期以來,科學家們一直在嘗試用類澱粉蛋白標靶藥物來減緩阿茲海默症,但未能成功。2021年,FDA在爭議中有條件核准了一種名為「阿杜卡努單抗」(Aduhelm)的藥物,但因缺乏證據表示該藥確實有效,此決定很快就以失敗告終。Leqembi的核准以及donanemab樂觀的數據,讓針對類澱粉蛋白堆積的療法再度成為顯學。
(台北時報林俐凱編譯)

A: The 23rd Taiwan Pride parade will be marching again on Saturday, Oct. 25. B: Will the parade kick off from Taipei City Hall Plaza as usual? A: Yup, and there will be over 110 LGBT-themed booths at the Rainbow Festival in the plaza. B: The organizer is reportedly teaming up with Japanese, South Korean and other international groups. A: So we are likely to see more foreign visitors from across the world. Hopefully, this year’s parade can smash the record of 200,000 marchers set in 2019. A: 第 23 屆台灣同志遊行本週六即將登場。 B: 遊行還是從台北市政府前廣場出發嗎? A: 對,廣場「彩虹市集」還有超過

California will phase out certain ultra-processed foods from school meals over the next decade under a first-in-the-nation law signed on Oct. 8 by Gov. Gavin Newsom. The law seeks to define ultra-processed foods, the often super-tasty products typically full of sugar, salt and unhealthy fats. The legislation requires the state’s Department of Public Health to adopt rules by mid-2028 defining “ultra-processed foods of concern” and “restricted school foods.” Schools have to start phasing out those foods by July 2029, and districts will be barred from selling them for breakfast or lunch by July 2035. Vendors will be banned from providing the “foods

Have you ever bought a new smartphone and suddenly found yourself dissatisfied with your perfectly fine headphones? Before long, you’ve purchased premium wireless earbuds, a protective case and a fast-charging station. What begins as a single acquisition snowballs into a shopping spree—this is the Diderot effect in action. Named after the 18th-century French philosopher Denis Diderot, the Diderot effect originates from an essay he wrote. In it, he recounted receiving a luxurious robe as a gift. As lovely as it was, the robe clashed with the rest of his humble belongings. One by one, he replaced his possessions to match the

A: As the Taiwan Pride parade enters its 23rd year, the nation also celebrates the sixth anniversary of the legalization of same-sex marriage. B: However, a poll showed that support for same-sex marriage slightly dropped to 54.3 percent from last year’s 56.5 percent. A: The government is wavering on whether to extend the Assisted Reproduction Act to same-sex couples, leading to public doubts. B: Since US President Donald Trump took office in January, his oppression of Diversity, Equity and Inclusion (DEI) programs has also frustrated the global LGBT community. A: Let’s join the parade in Taipei tomorrow to