A panel of experts convened by the US Food and Drug Administration (FDA) on Wednesday unanimously recommended COVID-19 vaccines for children younger than five, the final age group awaiting immunization in most countries.
Formal authorizations for Moderna and Pfizer should follow soon, with the first shots in arms expected early next week, just more than a year and a half after the first COVID-19 vaccines were greenlighted for elderly people in December 2020.
In Taipei, the Central Epidemic Command Center (CECC) yesterday said that the nation’s Food and Drug Administration is considering granting emergency use authorization (EUA) to the Moderna COVID-19 vaccine for ages six months to five years.

Photo: Wang Yi-sung, Taipei Times
If approved, the center would ask the Advisory Committee on Immunization Practices to discuss the issue, said Centers for Disease Control Deputy Director-General Philip Lo (羅一鈞), deputy head of the CECC’s medical response division.
Unlike regulators in other countries, the US FDA offers livestreams of its internal deliberations and its stamp of approval is considered the global gold standard.
Opening the discussion, senior FDA scientist Peter Marks said that despite studies showing that the majority of children have now been infected with COVID-19, the high rate of hospitalizations among infants, toddlers and young children during last winter’s Omicron wave of SARS-CoV-2 underscored an urgent need for vaccination.

Photo courtesy of the CECC
“We are dealing with an issue where we have to be careful we don’t become numb to the pediatric deaths because of the overwhelming number of older deaths,” he said.
“Every life is important and vaccine-preventable deaths are something we would like to try to do something about,” he said.
The US has recorded 480 COVID-19 deaths in the 0-to-4 age group in the COVID-19 pandemic — far higher than even a bad flu season, Marks said.
As of last month, there had been 45,000 hospitalizations in that group, nearly one-quarter of which required intensive care.
Ahead of the meeting, the FDA posted its independent analyses of the pharmaceutical firms’ vaccines, deeming both safe and effective.
Both vaccines are based on messenger RNA, which delivers genetic code for the coronavirus spike protein to human cells that then grow it on their surface, training the immune system to be ready.
Pfizer sought authorization for three doses at 3 micrograms given to children aged six months to four years, while Moderna asked for the FDA to authorize its vaccine as two doses of a higher 25 micrograms for ages six months to five years.
Both vaccines were tested in trials of thousands of children. They were found to cause similar levels of mild side effects as in older age groups and triggered similar levels of antibodies.
There are about 20 million US children aged four years and under. Although obesity, neurological disorders and asthma are associated with increased risk of severe disease among young children, it is not easy to predict severe outcomes. Data show that 64 percent of hospitalizations in those under five occurred in patients without comorbidities.
Children can also go on to contract multisystem inflammatory syndrome in children (MIS-C), a rare, but serious post-viral condition. About 3 to 6 percent can experience long COVID symptoms for more than 12 weeks.
The FDA is expected to soon act on the panel’s recommendation, and the matter will go to the US Centers for Disease Control and Prevention for a final say.
White House officials last week said the rollout of 10 million shots at pharmacies and doctors’ offices could begin as soon as Tuesday next week.
Taiwan’s FDA on April 17 issued an EUA for Moderna, at 50 micrograms per dose, for children aged six to 11, and on April 21 issued an EUA for the Pfizer-
BioNTech COVID-19 vaccine, at 10 micrograms per dose, for children aged five to 11.
Inoculations of children aged six to 11 with the Moderna vaccine began on May 2, followed by the Pfizer-BioNTech vaccine on May 25.
Additional reporting by By Wu Liang-yi and Jake Chung
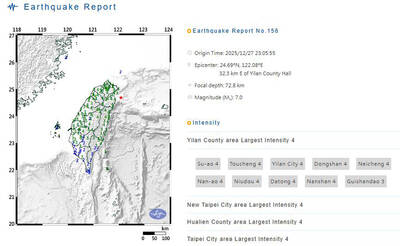
A magnitude 7.0 earthquake struck off Yilan at 11:05pm yesterday, the Central Weather Administration (CWA) said. The epicenter was located at sea, about 32.3km east of Yilan County Hall, at a depth of 72.8km, CWA data showed There were no immediate reports of damage. The intensity of the quake, which gauges the actual effect of a seismic event, measured 4 in Yilan County area on Taiwan’s seven-tier intensity scale, the data showed. It measured 4 in other parts of eastern, northern and central Taiwan as well as Tainan, and 3 in Kaohsiung and Pingtung County, and 2 in Lienchiang and Penghu counties and 1

FOREIGN INTERFERENCE: Beijing would likely intensify public opinion warfare in next year’s local elections to prevent Lai from getting re-elected, the ‘Yomiuri Shimbun’ said Internal documents from a Chinese artificial intelligence (AI) company indicated that China has been using the technology to intervene in foreign elections, including propaganda targeting Taiwan’s local elections next year and presidential elections in 2028, a Japanese newspaper reported yesterday. The Institute of National Security of Vanderbilt University obtained nearly 400 pages of documents from GoLaxy, a company with ties to the Chinese government, and found evidence that it had apparently deployed sophisticated, AI-driven propaganda campaigns in Hong Kong and Taiwan to shape public opinion, the Yomiuri Shimbun reported. GoLaxy provides insights, situation analysis and public opinion-shaping technology by conducting network surveillance

‘POLITICAL GAME’: DPP lawmakers said the motion would not meet the legislative threshold needed, and accused the KMT and the TPP of trivializing the Constitution The Legislative Yuan yesterday approved a motion to initiate impeachment proceedings against President William Lai (賴清德), saying he had undermined Taiwan’s constitutional order and democracy. The motion was approved 61-50 by lawmakers from the main opposition Chinese Nationalist Party (KMT) and the smaller Taiwan People’s Party (TPP), who together hold a legislative majority. Under the motion, a roll call vote for impeachment would be held on May 19 next year, after various hearings are held and Lai is given the chance to defend himself. The move came after Lai on Monday last week did not promulgate an amendment passed by the legislature that

Taiwan is gearing up to celebrate the New Year at events across the country, headlined by the annual countdown and Taipei 101 fireworks display at midnight. Many of the events are to be livesteamed online. See below for lineups and links: Taipei Taipei’s New Year’s Party 2026 is to begin at 7pm and run until 1am, with the theme “Sailing to the Future.” South Korean girl group KARA is headlining the concert at Taipei City Hall Plaza, with additional performances by Amber An (安心亞), Nick Chou (周湯豪), hip-hop trio Nine One One (玖壹壹), Bii (畢書盡), girl group Genblue (幻藍小熊) and more. The festivities are to