Medigen Vaccine Biologics Corp’s (高端疫苗) phase 3 clinical trials for an enterovirus 71 (EV71) vaccine completed the multi-regional, multi-central data “unblinding” yesterday, with the results, including safety, immunogenicity and efficacy, meeting its expectations.
The company would compile a final analysis report as soon as possible, and apply for the new drug certificate from domestic and foreign drug authorities in the third quarter, Medigen said in a Taiwan Stock Exchange filing yesterday.
Its phase 3 trials were conducted in Taiwan and Vietnam with 3,049 participants ranging from two months old to six years old, Medigen said.

Photo: Sam Yeh, AFP
The company enrolled its first participant for the phase 3 trials in April 2019 and completed the enrollment of all participants in December that year, it said.
Participants were recruited in a randomized, double-blinded and placebo-controlled study, it added.
Participants were divided into three age groups: two months to six months old, six months to two years old, and two years to six years old, Medigen said.
The number of infants from two months to six months old accounted for 25 percent of all participants, it said.
The results showed the drug’s safety and tolerance were good, even for young participants, and there was no significant difference in safety and tolerance between participants who were given the vaccine and those who received a placebo, the company said.
As for immunogenicity, results showed that the seroprotection rates 28 days, six months and one year after two EV71 vaccination doses were higher than health authorities’ requirements, it said. The seroprotection rates refer to the degree of antibody prevalence.
In terms of efficacy, Medigen said that the experimental drug offered statistically significant protection against the enterovirus under the Poisson regression model.
As enterovirus infections are popular in Taiwan, China and Southeast Asia, the company hopes its vaccine can hit the market soon, as there are no EV71 vaccine products available in other parts of the world, except for China.
At present, only three Chinese manufacturers have launched enterovirus vaccines to be marketed locally in China, Medigen said.
Those vaccines are suitable for children over the age of six months, it said.
In 2018, the market sales of China’s EV71 vaccines reached more than NT$30 billion (US$1.08 billion), and about 21 million doses were sold per year on average in the past three years, Medigen said.
The company on June 10 reported positive results from an interim analysis of phase 2 trials for its COVID-19 vaccine. It has applied to the Food and Drug Administration for emergency use authorization and is preparing to conduct phase 3 trials abroad.
NOT JUSTIFIED: The bank’s governor said there would only be a rate cut if inflation falls below 1.5% and economic conditions deteriorate, which have not been detected The central bank yesterday kept its key interest rates unchanged for a fifth consecutive quarter, aligning with market expectations, while slightly lowering its inflation outlook amid signs of cooling price pressures. The move came after the US Federal Reserve held rates steady overnight, despite pressure from US President Donald Trump to cut borrowing costs. Central bank board members unanimously voted to maintain the discount rate at 2 percent, the secured loan rate at 2.375 percent and the overnight lending rate at 4.25 percent. “We consider the policy decision appropriate, although it suggests tightening leaning after factoring in slackening inflation and stable GDP growth,”

DIVIDED VIEWS: Although the Fed agreed on holding rates steady, some officials see no rate cuts for this year, while 10 policymakers foresee two or more cuts There are a lot of unknowns about the outlook for the economy and interest rates, but US Federal Reserve Chair Jerome Powell signaled at least one thing seems certain: Higher prices are coming. Fed policymakers voted unanimously to hold interest rates steady at a range of 4.25 percent to 4.50 percent for a fourth straight meeting on Wednesday, as they await clarity on whether tariffs would leave a one-time or more lasting mark on inflation. Powell said it is still unclear how much of the bill would fall on the shoulders of consumers, but he expects to learn more about tariffs

Greek tourism student Katerina quit within a month of starting work at a five-star hotel in Halkidiki, one of the country’s top destinations, because she said conditions were so dire. Beyond the bad pay, the 22-year-old said that her working and living conditions were “miserable and unacceptable.” Millions holiday in Greece every year, but its vital tourism industry is finding it harder and harder to recruit Greeks to look after them. “I was asked to work in any department of the hotel where there was a need, from service to cleaning,” said Katerina, a tourism and marketing student, who would
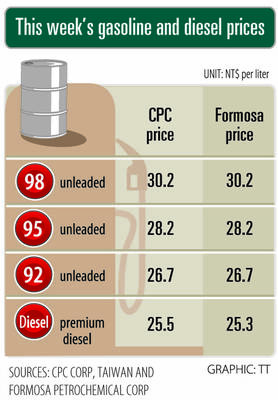
i Gasoline and diesel prices at fuel stations are this week to rise NT$0.1 per liter, as tensions in the Middle East pushed crude oil prices higher last week, CPC Corp, Taiwan (台灣中油) and Formosa Petrochemical Corp (台塑石化) said yesterday. International crude oil prices last week rose for the third consecutive week due to an escalating conflict between Israel and Iran, as the market is concerned that the situation in the Middle East might affect crude oil supply, CPC and Formosa said in separate statements. Front-month Brent crude oil futures — the international oil benchmark — rose 3.75 percent to settle at US$77.01