Two more Chinese drugmakers have said that a blood-pressure medication they exported to Taiwan contained a potentially cancer-causing impurity, a month after the same problem at another Chinese manufacturer prompted a global recall.
Tianyu Pharm and Rundu Pharma said batches of valsartan, an active ingredient widely used in medications for high blood pressure and congestive heart failure, contained N-nitrosodimethylamine (NDMA), which is believed to potentially cause cancer through long-term use.
Tianyu Pharm made its announcement yesterday in a statement to the Shenzhen Stock Exchange, where both companies are listed, while Rundu said the same in an exchange statement on Friday.
Tianyu Pharm said the problem was caught before its valsartan was distributed to end users in Taiwan, while Rundu Pharma said its products made it onto Taiwan’s market, but would be recalled.
The Food and Drug Administration on Thursday ordered a recall of 24.21 million tablets of heart medicine valsartan supplied by Zhuhai Rundu Pharmaceutical Co were found to be tainted.
The recall was instigated after it was discovered that Zhuhai’s valsartan contained NDMA.
The four types of medicines affected are the Valsart FC Tab 160mg, Valsart FC Tab 80 mg, Asartan FC Tab 5/80mg and the Valsart-H FC Tablets 80/12.5mg, which is produced by Standard Chem & Pharm Co in Taiwan.
More than 10 million tablets from six types of valsartan medicine supplied by Zhejiang Huahai Pharmaceuticals were recalled last month after they were found to be tainted by NDMA.

Taiwan is to commence mass production of the Tien Kung (天弓, “Sky Bow”) III, IV and V missiles by the second quarter of this year if the legislature approves the government’s NT$1.25 trillion (US$39.78 billion) special defense budget, an official said yesterday. Commenting on condition of anonymity, a defense official with knowledge of the matter said that the advanced systems are expected to provide crucial capabilities against ballistic and cruise missiles for the proposed “T-Dome,” an advanced, multi-layered air defense network. The Tien Kung III is an air defense missile with a maximum interception altitude of 35km. The Tien Kung IV and V
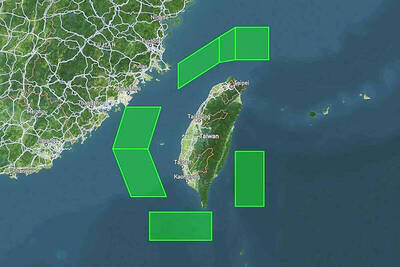
The disruption of 941 flights in and out of Taiwan due to China’s large-scale military exercises was no accident, but rather the result of a “quasi-blockade” used to simulate creating the air and sea routes needed for an amphibious landing, a military expert said. The disruptions occurred on Tuesday and lasted about 10 hours as China conducted live-fire drills in the Taiwan Strait. The Civil Aviation Administration (CAA) said the exercises affected 857 international flights and 84 domestic flights, affecting more than 100,000 travelers. Su Tzu-yun (蘇紫雲), a research fellow at the government-sponsored Institute for National Defense and Security Research, said the air

A strong continental cold air mass is to bring pollutants to Taiwan from tomorrow, the Ministry of Environment said today, as it issued an “orange” air quality alert for most of the country. All of Taiwan except for Hualien and Taitung counties is to be under an “orange” air quality alert tomorrow, indicating air quality that is unhealthy for sensitive groups. In China, areas from Shandong to Shanghai have been enveloped in haze since Saturday, the ministry said in a news release. Yesterday, hourly concentrations of PM2.5 in these areas ranged from 65 to 160 micrograms per cubic meter (mg/m³), and pollutants were

Taiwan lacks effective and cost-efficient armaments to intercept rockets, making the planned “T-Dome” interception system necessary, two experts said on Tuesday. The concerns were raised after China’s military fired two waves of rockets during live-fire drills around Taiwan on Tuesday, part of two-day exercises code-named “Justice Mission 2025.” The first wave involved 17 rockets launched at 9am from Pingtan in China’s Fujian Province, according to Lieutenant General Hsieh Jih-sheng (謝日升) of the Office of the Deputy Chief of the General Staff for Intelligence at the Ministry of National Defense. Those rockets landed 70 nautical miles (129.6km) northeast of Keelung without flying over Taiwan,