More than 1.78 million tablets of an antipsychotic medication have been recalled due to cross-contamination of pharmaceutical ingredients during the manufacturing process, the Food and Drug Administration (FDA) said on Tuesday.
A batch of 5mg Otsuka Abilify (aripiprazole) tablets, or more than 1.43 million doses, and two batches of Otsuka Ability (aripiprazole) 30mg tablets, or about 354,410 doses, have been recalled, the FDA said.
The medication is used to treat several mental health conditions, including schizophrenia, bipolar disorder, autism spectrum disorder and Tourette’s syndrome.
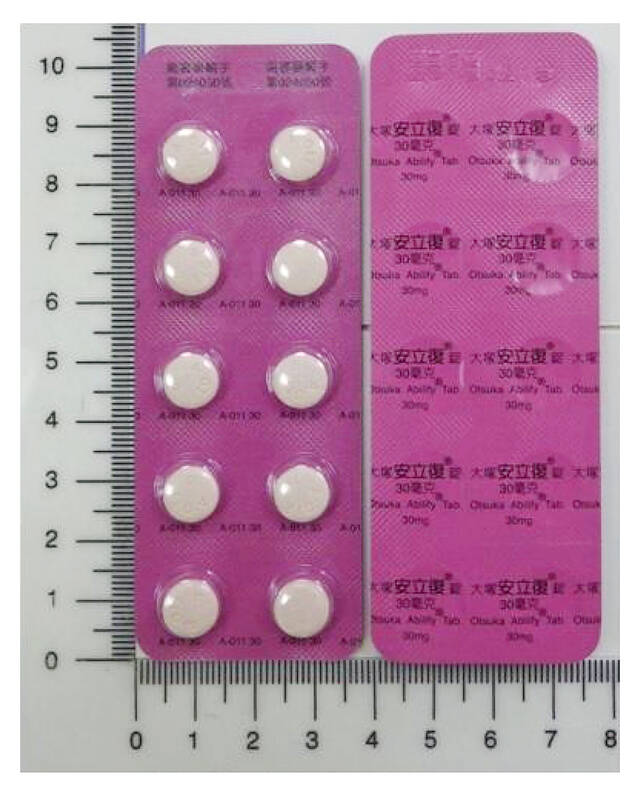
Photo courtesy of the Food and Drug Administration
On April 25, routine monitoring of international alerts showed that the US Food and Drug Administration issued a recall for the two dosage forms of Otsuka Ability (aripiprazole) due to cross-contamination from extremely small amounts of an active pharmaceutical ingredient during the manufacturing process, FDA Deputy Director-General Wang Te-yuan (王德原) said.
Insufficient cleaning of equipment at the production site was the likely cause, Wang said.
The two forms of the medication are widely used in Taiwan.
More than 7.59 million 5mg tablets were used in Taiwan in 2022, while NHI reimbursement applications tallied about 238,000 30mg tablets, a National Health Insurance Administration report said last year.
Aripiprazole is an active ingredient used to treat schizophrenia in adults and adolescents, and for bipolar disorder in children aged 10 to 17, the FDA said.
It is also used to treat irritability associated with autistic disorder in pediatric patients aged six to 17, it said.
Separately, the FDA said that fresh strawberries from Japan would remain subject to batch-by-batch border inspections after a new shipment was found to contain a pesticide that is banned in Taiwan.
The shipment of 24kg of strawberries from Momofuku Shoji Co would either be returned to the country of origin or destroyed, it said.
Sample testing on April 17 found that the strawberries contained 0.04 parts per million of acrinathrin, a synthetic pyrethroid that has a high insecticidal activity against a range of insects, including mites.
Acrinathrin is a banned pesticide that cannot be used on strawberries in Taiwan, FDA Deputy Director-General Lin Chin-fu (林金富) said.
Of the 812 batches of Japanese strawberries inspected from Oct. 29 last year to April 29, 32 failed to meet Taiwan’s safety standards, mostly because they were found to have excessive levels of pesticide residue, Lin said.
Since June 1 last year, fresh strawberries from Japan have been subject to 100 percent checks at the border, a measure that was supposed to be lifted at the end of last month, Lin said.
However, because shipments of strawberries continue to fail safety inspections, every shipment of imported Japanese strawberries would be inspected until Dec. 31, he said.

China might accelerate its strategic actions toward Taiwan, the South China Sea and across the first island chain, after the US officially entered a military conflict with Iran, as Beijing would perceive Washington as incapable of fighting a two-front war, a military expert said yesterday. The US’ ongoing conflict with Iran is not merely an act of retaliation or a “delaying tactic,” but a strategic military campaign aimed at dismantling Tehran’s nuclear capabilities and reshaping the regional order in the Middle East, said National Defense University distinguished adjunct lecturer Holmes Liao (廖宏祥), former McDonnell Douglas Aerospace representative in Taiwan. If

Prosecutors in New Taipei City yesterday indicted 31 individuals affiliated with the Chinese Nationalist Party (KMT) for allegedly forging thousands of signatures in recall campaigns targeting three Democratic Progressive Party (DPP) lawmakers. The indictments stem from investigations launched earlier this year after DPP lawmakers Su Chiao-hui (蘇巧慧) and Lee Kuen-cheng (李坤城) filed criminal complaints accusing campaign organizers of submitting false signatures in recall petitions against them. According to the New Taipei District Prosecutors Office, a total of 2,566 forged recall proposal forms in the initial proposer petition were found during the probe. Among those
ECHOVIRUS 11: The rate of enterovirus infections in northern Taiwan increased last week, with a four-year-old girl developing acute flaccid paralysis, the CDC said Two imported cases of chikungunya fever were reported last week, raising the total this year to 13 cases — the most for the same period in 18 years, the Centers for Disease Control (CDC) said yesterday. The two cases were a Taiwanese and a foreign national who both arrived from Indonesia, CDC Epidemic Intelligence Center Deputy Director Lee Chia-lin (李佳琳) said. The 13 cases reported this year are the most for the same period since chikungunya was added to the list of notifiable communicable diseases in October 2007, she said, adding that all the cases this year were imported, including 11 from

The Ma-anshan Nuclear Power Plant’s license has expired and it cannot simply be restarted, the Executive Yuan said today, ahead of national debates on the nuclear power referendum. The No. 2 reactor at the Ma-anshan Nuclear Power Plant in Pingtung County was disconnected from the nation’s power grid and completely shut down on May 17, the day its license expired. The government would prioritize people’s safety and conduct necessary evaluations and checks if there is a need to extend the service life of the reactor, Executive Yuan spokeswoman Michelle Lee (李慧芝) told a news conference. Lee said that the referendum would read: “Do