The National Health Insurance Administration (NHIA) urged people who will finish their repeat prescription during the seven-day Lunar New Year holiday to refill their prescriptions in the 10 days before the holiday, starting on Monday next week.
As most healthcare facilities are to reduce or suspend outpatient clinics and medicine dispensing services during the Lunar New Year holiday — from Feb. 8 to Feb. 14 — the NHIA on Tuesday said rules for refilling repeat prescriptions would be eased, allowing patients to book a follow-up appointment for new prescriptions or refill their repeat prescriptions earlier than the rules require.
NHIA Director-General Shih Chung-liang (石崇良) said that according to the Regulations Governing the National Health Insurance Medical Care (全民健康保險醫療辦法), repeat prescriptions are for people with stable long-term conditions to receive regular treatment, and the validity period of a repeat prescription is a maximum of 90 days from when the prescription is issued.

Photo: Chiu Chih-jou, Taipei Times
The medication is not dispensed all at once, as the maximum limit of each refill is 30 days, he said, adding that people can only refill their prescription in the 10 days before the medication they previously received has been used up.
Therefore, to prevent the discontinuation of medication, the agency is reminding people to refill their repeat prescriptions or get a new prescription before the Lunar New Year holiday.
Separately, the Food and Drug Administration (FDA) earlier this month said that as many families clean the house before the Lunar New Year holiday, it urged people to dispose of unused or expired drugs, and to store drugs properly.
The FDA said that people should carefully check the drugs they have at home and dispose of those that are unused after a course of treatment, expired or spoiled over-the-counter drugs — including those that have changed color, been crushed or dissolved — and drugs where the packaging have been open too long.
It said the stability of some medications decrease once the original packaging is opened, even before the expiration date, and they should be disposed of, such as pills in bottles opened more than six months ago, eye drops or ointment opened more than a month ago, or any medication where the packaging has been opened for an unknown length of time.
Specialty drugs — injections, anti-cancer drugs, antibiotics, hormone drugs and controlled medication — should be returned to healthcare facilities or pharmacies for recycling, the FDA said.
General medicines can be directly thrown into trash bags and incinerated with general waste, it said, adding that liquid medicine should not be poured into the toilet or sink, but instead should be absorbed with soil, tea leaves, newspaper, coffee grounds and sealed in a plastic bag, then disposed of as general waste.
The FDA also reminded people to store medication according to the packaging or the pharmacist’s instruction and not to randomly put drugs into the refrigerator unless indicated.
It suggested writing the date the package was opened on the packaging, and to seal it properly after each use.
Medicine should always be stored out of the sight and reach of children, it added.
The public can also consult with a pharmacist and prepare any medication required before traveling, such as motion sickness medicine.
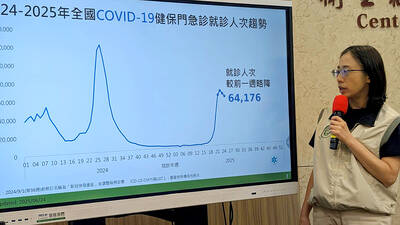
ECHOVIRUS 11: The rate of enterovirus infections in northern Taiwan increased last week, with a four-year-old girl developing acute flaccid paralysis, the CDC said Two imported cases of chikungunya fever were reported last week, raising the total this year to 13 cases — the most for the same period in 18 years, the Centers for Disease Control (CDC) said yesterday. The two cases were a Taiwanese and a foreign national who both arrived from Indonesia, CDC Epidemic Intelligence Center Deputy Director Lee Chia-lin (李佳琳) said. The 13 cases reported this year are the most for the same period since chikungunya was added to the list of notifiable communicable diseases in October 2007, she said, adding that all the cases this year were imported, including 11 from

Prosecutors in New Taipei City yesterday indicted 31 individuals affiliated with the Chinese Nationalist Party (KMT) for allegedly forging thousands of signatures in recall campaigns targeting three Democratic Progressive Party (DPP) lawmakers. The indictments stem from investigations launched earlier this year after DPP lawmakers Su Chiao-hui (蘇巧慧) and Lee Kuen-cheng (李坤城) filed criminal complaints accusing campaign organizers of submitting false signatures in recall petitions against them. According to the New Taipei District Prosecutors Office, a total of 2,566 forged recall proposal forms in the initial proposer petition were found during the probe. Among those

The Mainland Affairs Council (MAC) today condemned the Chinese Communist Party (CCP) after the Czech officials confirmed that Chinese agents had surveilled Vice President Hsiao Bi-khim (蕭美琴) during her visit to Prague in March last year. Czech Military Intelligence director Petr Bartovsky yesterday said that Chinese operatives had attempted to create the conditions to carry out a demonstrative incident involving Hsiao, going as far as to plan a collision with her car. Hsiao was vice president-elect at the time. The MAC said that it has requested an explanation and demanded a public apology from Beijing. The CCP has repeatedly ignored the desires

The Ma-anshan Nuclear Power Plant’s license has expired and it cannot simply be restarted, the Executive Yuan said today, ahead of national debates on the nuclear power referendum. The No. 2 reactor at the Ma-anshan Nuclear Power Plant in Pingtung County was disconnected from the nation’s power grid and completely shut down on May 17, the day its license expired. The government would prioritize people’s safety and conduct necessary evaluations and checks if there is a need to extend the service life of the reactor, Executive Yuan spokeswoman Michelle Lee (李慧芝) told a news conference. Lee said that the referendum would read: “Do