The Philippines yesterday ordered a probe into the immunization of more than 730,000 children with a vaccine for dengue that has been suspended following an announcement by French drug company Sanofi that it could worsen the disease in some cases.
A non-governmental organization in the Philippines said it had received information that three children who were vaccinated with Dengvaxia in April last year had died, but Sanofi said no deaths had been reported as a result of the program, adding that it is working to resolve the fears.
“As far as we know, as far as we are made aware, there are no reported deaths that are related to dengue vaccination,” Sanofi Pasteur Philippines medical director Ruby Dizon told a news conference in Manila.
Photo: Reuters
Last week, the Philippine Department of Health halted the use of Dengvaxia after Sanofi said it must be strictly limited due to evidence it can worsen the disease in people not previously exposed to the infection.
In a statement issued in the Philippines, Sanofi explained the “new findings,” but said that the long-term safety evaluation of the vaccines showed significantly fewer hospitalizations due to dengue in vaccinated people over nine years old compared with those who had not been vaccinated.
Those stricken with dengue in a study all had fully recovered, Sanofi Pasteur global medical head Ng Su-Peing said yesterday.
Nearly 734,000 children aged nine and over in the Philippines have received one dose of the vaccine as part of a program that cost 3.5 billion pesos (US$69.54 million).
The Philippine Department of Justice yesterday ordered the Philippine National Bureau of Investigation to look into “the alleged danger to public health ... and if evidence so warrants, to file appropriate charges thereon.”
There was no indication that Philippine health officials knew of any risks when they administered the vaccination.
However, the WHO in a paper in July last year said that “vaccination may be ineffective or may theoretically even increase the future risk of hospitalized or severe dengue illness in those who are seronegative at the time of first vaccination regardless of age.”
Singaporean Health Sciences Authority last week said that it flagged risks when the vaccine was approved there in October last year and was working with Sanofi to strengthen risk warnings on the drug’s packaging.
According to Sanofi in Manila, 19 licenses were granted for Dengvaxia, and it was launched in 11 countries, only two of which — the Philippines and Brazil — had public programs to administer the vaccine.
A spokesman for Philippine President Rodrigo Duterte on Sunday said the government would hold to account those responsible for a program that had placed thousands of lives at risk.
“We will leave no stone unturned in making those responsible for this shameless public health scam, which puts hundreds of thousands of young lives at risk, accountable,” spokesman Harry Roque said in a statement.
There had been no reported case of “severe dengue infection” since the vaccine was administered, Roque said, calling on the public “not to spread information that may cause undue alarm.”
However, Volunteers Against Crime and Corruption said it was checking a report that three children on the northern island of Luzon had died since being vaccinated in April last year and described the situation as a disaster.
Additional reporting by AP

‘IN A DIFFERENT PLACE’: The envoy first visited Shanghai, where he attended a Chinese basketball playoff match, and is to meet top officials in Beijing tomorrow US Secretary of State Antony Blinken yesterday arrived in China on his second visit in a year as the US ramps up pressure on its rival over its support for Russia while also seeking to manage tensions with Beijing. The US diplomat tomorrow is to meet China’s top brass in Beijing, where he is also expected to plead for restraint as Taiwan inaugurates president-elect William Lai (賴清德), and to raise US concerns on Chinese trade practices. However, Blinken is also seeking to stabilize ties, with tensions between the world’s two largest economies easing since his previous visit in June last year. At the
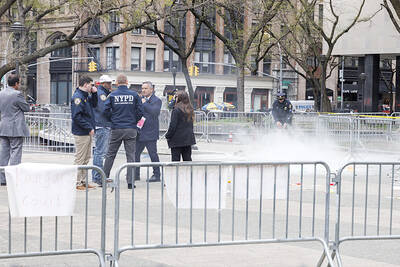
UNSETTLING IMAGES: The scene took place in front of TV crews covering the Trump trial, with a CNN anchor calling it an ‘emotional and unbelievably disturbing moment’ A man who doused himself in an accelerant and set himself on fire outside the courthouse where former US president Donald Trump is on trial has died, police said yesterday. The New York City Police Department (NYPD) said the man was declared dead by staff at an area hospital. The man was in Collect Pond Park at about 1:30pm on Friday when he took out pamphlets espousing conspiracy theories, tossed them around, then doused himself in an accelerant and set himself on fire, officials and witnesses said. A large number of police officers were nearby when it happened. Some officers and bystanders rushed

Beijing is continuing to commit genocide and crimes against humanity against Uyghurs and other Muslim minorities in its western Xinjiang province, U.S. Secretary of State Antony Blinken said in a report published on Monday, ahead of his planned visit to China this week. The State Department’s annual human rights report, which documents abuses recorded all over the world during the previous calendar year, repeated language from previous years on the treatment of Muslims in Xinjiang, but the publication raises the issue ahead of delicate talks, including on the war in Ukraine and global trade, between the top U.S. diplomat and Chinese

RIVER TRAGEDY: Local fishers and residents helped rescue people after the vessel capsized, while motorbike taxis evacuated some of the injured At least 58 people going to a funeral died after their overloaded river boat capsized in the Central African Republic’s (CAR) capital, Bangui, the head of civil protection said on Saturday. “We were able to extract 58 lifeless bodies,” Thomas Djimasse told Radio Guira. “We don’t know the total number of people who are underwater. According to witnesses and videos on social media, the wooden boat was carrying more than 300 people — some standing and others perched on wooden structures — when it sank on the Mpoko River on Friday. The vessel was heading to the funeral of a village chief in