US regulators on Friday told a New York fertility doctor to stop marketing an experimental procedure that uses DNA from three people — a mother, a father and an egg donor — to avoid certain genetic diseases.
The doctor, John Zhang, used the technique to help a Jordanian couple have a baby boy last year.
According to the US Food and Drug Administration (FDA), Zhang said his companies would not use the technology in the US again without permission, yet they continue to promote it.
The procedure is not approved in the US and the US Congress has barred the FDA from even reviewing proposals to conduct such experiments.
A receptionist at Zhang’s New Hope Fertility Clinic in New York said on Friday that no one was available to comment.
Zhang heads the clinic and a related company, Darwin Life Inc.
New Hope’s Web site touts having achieved the “first live birth” using the technology, along with other advanced fertility treatments it offers.
The FDA’s letter to Zhang cites several other marketing claims, including a reference to “the first proven treatment for certain genetic disorders.”
The birth of the boy was disclosed in September last year.
The mother carries DNA that could have given her child Leigh syndrome, a severe neurological disorder that usually kills within a few years of birth.
The experimental technique involves removing some of the mother’s DNA from an egg and leaving the disease-causing DNA behind. The healthy DNA gets slipped into a donor’s egg, which is then fertilized.
As a result, the baby inherits DNA from both parents and the egg donor, producing what has been called “three-parent babies” — although the DNA contribution from the egg donor is small.
People carry DNA in two places, the nucleus of the cell and in structures called mitochondria, which lie outside the nucleus. The technique is designed to transfer only DNA of the nucleus to the donor egg.
A medical journal report on the case said the procedure was done at the New York clinic and the embryo was taken to Mexico, where it was implanted.
The procedure is not illegal in Mexico.
Last year, a report from a panel of US government advisers said it is ethical to begin testing this approach in pregnancy, as long as the first studies follow strict safety steps.
The studies must include women at high risk of passing on a severe disease and, at first, implant only male embryos, so the alterations would not pass to future generations.

‘IN A DIFFERENT PLACE’: The envoy first visited Shanghai, where he attended a Chinese basketball playoff match, and is to meet top officials in Beijing tomorrow US Secretary of State Antony Blinken yesterday arrived in China on his second visit in a year as the US ramps up pressure on its rival over its support for Russia while also seeking to manage tensions with Beijing. The US diplomat tomorrow is to meet China’s top brass in Beijing, where he is also expected to plead for restraint as Taiwan inaugurates president-elect William Lai (賴清德), and to raise US concerns on Chinese trade practices. However, Blinken is also seeking to stabilize ties, with tensions between the world’s two largest economies easing since his previous visit in June last year. At the
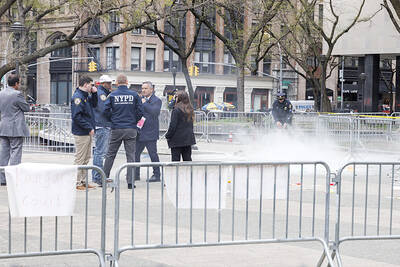
UNSETTLING IMAGES: The scene took place in front of TV crews covering the Trump trial, with a CNN anchor calling it an ‘emotional and unbelievably disturbing moment’ A man who doused himself in an accelerant and set himself on fire outside the courthouse where former US president Donald Trump is on trial has died, police said yesterday. The New York City Police Department (NYPD) said the man was declared dead by staff at an area hospital. The man was in Collect Pond Park at about 1:30pm on Friday when he took out pamphlets espousing conspiracy theories, tossed them around, then doused himself in an accelerant and set himself on fire, officials and witnesses said. A large number of police officers were nearby when it happened. Some officers and bystanders rushed

Beijing is continuing to commit genocide and crimes against humanity against Uyghurs and other Muslim minorities in its western Xinjiang province, U.S. Secretary of State Antony Blinken said in a report published on Monday, ahead of his planned visit to China this week. The State Department’s annual human rights report, which documents abuses recorded all over the world during the previous calendar year, repeated language from previous years on the treatment of Muslims in Xinjiang, but the publication raises the issue ahead of delicate talks, including on the war in Ukraine and global trade, between the top U.S. diplomat and Chinese

RIVER TRAGEDY: Local fishers and residents helped rescue people after the vessel capsized, while motorbike taxis evacuated some of the injured At least 58 people going to a funeral died after their overloaded river boat capsized in the Central African Republic’s (CAR) capital, Bangui, the head of civil protection said on Saturday. “We were able to extract 58 lifeless bodies,” Thomas Djimasse told Radio Guira. “We don’t know the total number of people who are underwater. According to witnesses and videos on social media, the wooden boat was carrying more than 300 people — some standing and others perched on wooden structures — when it sank on the Mpoko River on Friday. The vessel was heading to the funeral of a village chief in