The Ministry of Health and Welfare will tighten controls over food companies that use advertisements to suggest that their products have therapeutic effects, or mislead people by labeling their products as “endorsed” or “recommended” by doctors, Minister of Health and Welfare Chen Shih-chung (陳時中) said on Wednesday.
Chen made the announcement during a question-and-answer session at the legislature’s Social Welfare and Environmental Hygiene Committee meeting in response to a question by Democratic Progressive Party (DPP) Legislator Lin Ching-yi (林靜儀), who is a gynecologist/obstetrician.
“We are outraged to see food companies taking advantage of information asymmetry and consumers’ feeling of insecurity to sell their products,” Lin said.

Photo: Wu Liang-yi, Taipei Times
Some companies advertise dietary supplements in ways that imply therapeutic effects, for example by having a doctor talk about a disease or healthcare, followed by an advertisement of the product, Lin said.
Lin held up a full-page newspaper ad of a herbal supplement run in January, which had the phrases: “Five major medical centers,” “Clinical experiments” and “Recommended by 300 gynecologists” in large, bold lettering.
She asked if the company has violated the law.
“Food products cannot be claimed to have therapeutic effects. Doctors are not supposed to endorse such products,” Chen said.
The legality of such advertisements has been ambiguous, “but we now consider them illegal,” Chen said.
Food and Drug Administration (FDA) Director-General Wu Shou-mei (吳秀梅) said food products cannot be labeled, promoted or advertised as having therapeutic effects, and those who do so may face a fine of between NT$600,000 and NT$5 million (US$19,633 and US$163,612).
The FDA last year fined 3,982 food companies a total of about NT$115 million over such violations, Wu added.
Lin said she contacted the Taiwan Association of Obstetrics and Gynecology and asked about the “300 gynecologists” who recommended the herbal supplement, after which the food company said the advertising company used the word “recommended” by mistake.
Wu said the food company has violated the law and might be fined.
When Lin asked whether doctors who endorse food products that claim to have therapeutic effects would also be punished, Chen said doctors would face consequences if they endorse such products in a professional capacity.
Lin said many food companies that sell “medicine-like” products continue to do so even after being fined multiple times, as the profits outstrip the fines.

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater
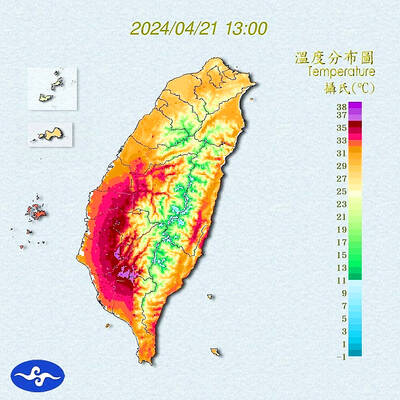
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching