The Food and Drug Administration (FDA) yesterday broke a pledge it made on March 25 to publish a draft regulation — requiring importers of Japanese food products to obtain an official country-of-origin certificate and a radiation assessment report — within two weeks, saying it is still undergoing administrative processing.
At a press conference in Taipei yesterday morning to release details on the agency’s inspections of imported goods last year, FDA Food Safety Division Director Pan Jyh-quan (潘志寬) said the agency was still communicating with Japan on the matter, and acknowledged the difficulty of promulgating and implementing the draft regulation by the promised deadline.
“The agency respects extemporaneous motions passed by lawmakers, but previous experience indicates that we must be realistic. If there still is consultation work to be done, then we shall continue to communicate with interested parties,” Pan said.
Pan was referring to an impromptu motion passed by Democratic Progressive Party (DPP) Legislator Lin Shu-fen (林淑芬) at a meeting of the legislature’s Social Welfare and Environmental Hygiene Committee on March 25, demanding that the FDA publish and promulgate the draft regulation within two weeks.
If passed, the draft regulation would require importers of nine categories of Japanese food items — fresh vegetables and fruit; frozen vegetables and fruit; fresh aquatic products; frozen aquatic products; baby formula; dairy products; seaweed; tea leaves; and drinking water — to provide both a country-of-origin certificate and a radiation assessment report issued by the Japanese government.
The FDA has been criticized for procrastinating over implementing the regulation, as it started seeking public opinion over the proposal in late October last year, a process that usually takes just two months.
On March 25, FDA Director-General Chiang Yu-mei (姜郁美) promised to publish the draft regulation by yesterday so that it could take effect in June.
After being pressed by lawmakers at a separate committee meeting on Monday last week, Chiang pledged to bring the regulation into effect the day it was promulgated, which was supposed to be yesterday.
Chiang could not be reached for comment by press time, but a report published by the Chinese-language United Daily News earlier yesterday quoted Chiang as saying the draft regulation is to be published tomorrow at the earliest.
Accusing the FDA of trampling on legislative motions, Lin said she would boycott the agency for as long as it takes.
“[Legislative Speaker] Wang Jin-pyng (王金平) also said today [Wednesday] during his ongoing visit to Japan that the Taiwanese government will base its decision regarding whether to lift its import ban on food products from five Japanese prefectures near the Fukushima Dai-ichi nuclear power plant on scientific techniques and test results,” Lin said.
Lin said the government was too cowardly to directly request Japan to carry out the same processes as it does for other countries — issuing country-of-origin certificates and radiation assessment reports for its foodstuffs.
“Is our government being colonized by Japan or what?” she added.

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater
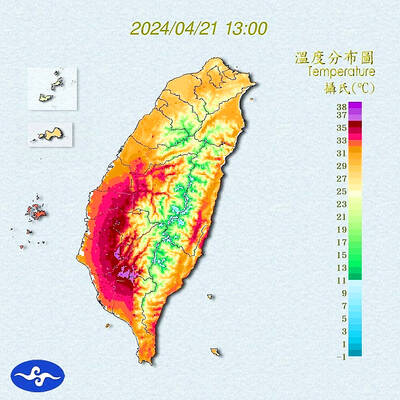
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching