The Food and Drug Administration (FDA) said yesterday that it has yet to ascertain whether medical examination equipment seller Medado Trading Co (鈦化企業) tampered with the expiry date on its cancer screening products more than once and how many hospitals it had sold the potentially problematic devices to.
“As Taipei-based Medado is only a sales agency rather than a licensed medical equipment manufacturer, we cannot track its sales records via the National Health Insurance program’s system and have to rely on local hospitals to double-check if they have ever purchased the screening tests that were allegedly tampered with,” FDA Division of Medical Devices and Cosmetics Section Chief Lue Tsai-luen (呂在綸) told a press conference in Taipei.
Lue was responding to a report in Chinese-language newspaper the Apple Daily yesterday which said that Medado’s proprietor, Wang Yu-ching (王裕景), and sales manager Kuan Pen-yi (官本義) have been charged by Taipei prosecutors with fraud, forgery and violation of the Pharmaceutical Affairs Act (藥事法).
It is alleged that the two changed expiration date labels on four kinds of cancer screening tests they procured from a registered pharmaceutical manufacturing company in 2011.
The tests included the CA19-9 screening test for pancreatic cancer, the alpha-fetoprotein (AFP) screening test for liver cancer, the prostate specific antigen (PSA) screening test for prostate cancer, and the serum free thyroxine (FT4) screening test for hyperthyroidism, the report said.
Citing an anonymous whistleblower, the report alleged that Wang and Kuan commissioned a company to attach a new label with an expiration date that was one month past the date on the original, to an entire batch of the tests it bought in 2011.
“The pair did so for the sole purpose of meeting the sales requirements in its contract with the Tri-Service General Hospital’s Songshan Branch, which said that the screening tests Medado provided must have more than six months remaining on their expiration dates,” the report quoted the informant as saying.
“However, the expiration date for the batch it purchased in 2011 was [allegedly] one month shorter than the required minimum shelf life. So they did what they did out of fear that the products might be rejected by the hospital and that they might be forced to shoulder the loss,” the informant was quoted as saying.
Lue said screening tests’ expiry dates are meant to offer guidance to medical practitioners on how long the tests would remain effective and accurate after production.
“That means an expired screening test could yield false positive or negative results, and therefore risks delaying cancer treatments that might be needed,” Lue said.
Lue said Medado’s allegedly illegal practice violated Article 84 of the Pharmaceutical Affairs Act, which carries a prison term of less than three years and a fine not exceeding NT$100,000 (US$3,300).
He also urged medical institutions that have had business dealings with Medado to check if the expiration date labels on products other than the screening tests in question have also been tampered with.
Later yesterday, Tri-Service General Hospital’s Songshan Branch dismissed speculation that it might have administered expired screening tests, saying that the problematic batch was used in its entirety less than one month after purchase.
“Our hospital orders cancer screening tests once every month because each batch purchased tends to be used up in less than 30 days,” the hospital said, adding that it bought screening tests from Medado from 2011 until it signed a sales contract with another agency in June this year.
The hospital said it would seek compensation from Medado after prosecutors conclude an investigation into whether the company had forged the tests’ expiry dates more than once.

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater
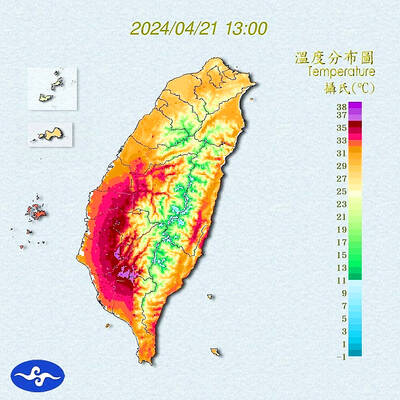
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching