Oral forms of chloramphenicol, an antibiotic, were formally withdrawn from Taiwan’s market starting yesterday, the Food and Drug Administration (FDA) said.
The agency said that compared with oral forms of chloramphenicol, which carries the risk of causing bone marrow suppression, safer substitutes now exist.
In addition, orally administered chloramphenicol is considered redundant because chloramphenicol injections, are now used for patients with serious infections, the agency said.
There are 66 approved drugs containing chloramphenicol, 36 of which were administered orally, the agency said.
Chloramphenicol is a broad-spectrum antibiotic, which means it is effective against infections caused by a wide variety of bacteria, and it inhibits bacterial protein synthesis, the agency said.
The agency’s anulment of the antibiotic’s permit comes after recent study results showed that the antibiotic is associated with the risks of hematologic toxicity and bone marrow suppression.
However, the benefits of the chloramphenicol injection for people who suffer drug-resistant bacterial infections still outweigh its risks, the agency said, and its continued clinical use has been approved.
It also works better at penetrating the central nervous system (CNS), an attribute that is needed for treating CNS infections.
Other forms of the antibiotic, such as eye drops, ear drops and suppositories, have also been permitted to be used clinically, said the health authority, as their therapeutic effects outweigh their relatively low health risks.
The US removed the oral form of chloramphenicol from its market in July 2012, the agency said.

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater
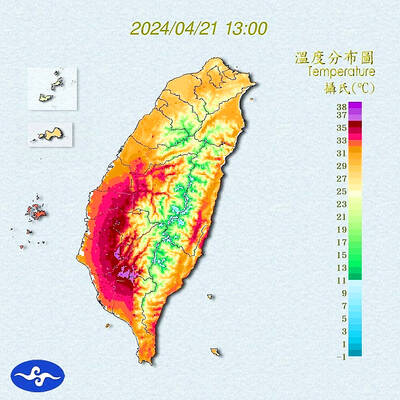
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching