New drug review processes would be accelerated and completed in 120 days in response to a US plan to raise pharmaceutical tariffs, the Ministry of Health and Welfare said yesterday.
Food and Drug Administration (FDA) Deputy Director-General Wang Der-yuan (王德原) yesterday told a news conference that measures have been taken to optimize new medicine review processes and help pharmaceutical companies obtain drug permit licenses as soon as possible.
Such processes normally take about 360 days to complete, if the new medicine is a new chemical entity (NCE) — a drug with new chemicals as principal components — or a drug made from biological sources, also known as biologics.

Photo: AP
However, NCEs or biologics considered important for pediatric treatments and serious diseases, as well as breakthrough therapies can have their review prioritized, with the process shortened to 240 days, he said.
If an NCE or a biologic has been approved in two of the US, the EU or Japan, it is qualified for a “type 1 express review” and can have its review completed as fast as in 180 days, Wang said.
If it has been approved in the three areas with identical data on chemistry, manufacturing and controls, it is qualified for a “type 2 express review” and its review process could be completed in 120 days, he added.
The ministry would also continue to encourage pharmaceutical companies to diversify sources of bulk drugs or active pharmaceutical ingredients so that they would not rely on a single supplier, he said.
China and India are the two major sources of bulk drugs used in Taiwan’s pharmaceutical industry, he said.
The FDA has requested pharmaceutical companies to evaluate whether they should find more bulk drug sources, Wang said, adding that the agency would prioritize the review of new medicines relying on a single source.
Pharmaceutical companies should also use domestically made bulk drugs, although they are mostly more expensive and less adopted, he said.
The administration has compiled an inventory of all US-made pharmaceutical products and sent a notice to their permit license holders to fully understand the medicine supply chain situation and properly boost domestic medicine stocks, he said.
It would also check stocks of primary medicines, such as anti-cancer drugs, orphan drugs and biologics to promptly initiate an investigation and evaluation whenever there is a potential shortage, he said.
The FDA would ask manufacturers of substitute medicines to increase production, or launch a drug import or manufacturing project, if necessary, he said.
Pharmaceutical companies can propose price adjustments to stabilize the market if changes in the global supply chain cause a surge in drug production costs, he said.
The ministry would continue to promote the application of generic drugs and biosimilars — biologics with similar structure and functions of a reference biologic made by another company — to bolster domestically made new medicines, Wang added.

The manufacture of the remaining 28 M1A2T Abrams tanks Taiwan purchased from the US has recently been completed, and they are expected to be delivered within the next one to two months, a source said yesterday. The Ministry of National Defense is arranging cargo ships to transport the tanks to Taiwan as soon as possible, said the source, who is familiar with the matter. The estimated arrival time ranges from late this month to early next month, the source said. The 28 Abrams tanks make up the third and final batch of a total of 108 tanks, valued at about NT$40.5 billion

Two Taiwanese prosecutors were questioned by Chinese security personnel at their hotel during a trip to China’s Henan Province this month, the Mainland Affairs Council (MAC) said yesterday. The officers had personal information on the prosecutors, including “when they were assigned to their posts, their work locations and job titles,” MAC Deputy Minister and spokesman Liang Wen-chieh (梁文傑) said. On top of asking about their agencies and positions, the officers also questioned the prosecutors about the Cross-Strait Joint Crime-Fighting and Judicial Mutual Assistance Agreement, a pact that serves as the framework for Taiwan-China cooperation on combating crime and providing judicial assistance, Liang
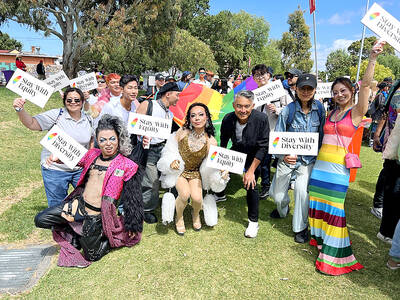
A group from the Taiwanese Designers in Australia association yesterday represented Taiwan at the Midsumma Pride March in Melbourne. The march, held in the St. Kilda suburb, is the city’s largest LGBTQIA+ parade and the flagship event of the annual Midsumma Festival. It attracted more than 45,000 spectators who supported the 400 groups and 10,000 marchers that participated this year, the association said. Taiwanese Designers said they organized a team to march for Taiwan this year, joining politicians, government agencies, professionals and community organizations in showing support for LGBTQIA+ people and diverse communities. As the first country in Asia to legalize same-sex

MOTIVES QUESTIONED The PLA considers Xi’s policies toward Taiwan to be driven by personal considerations rather than military assessment, the Epoch Times reports Chinese President Xi Jinping’s (習近平) latest purge of the Chinese People’s Liberation Army (PLA) leadership might have been prompted by the military’s opposition to plans of invading Taiwan, the Epoch Times said. The Chinese military opposes waging war against Taiwan by a large consensus, putting it at odds with Xi’s vision, the Falun Gong-affiliated daily said in a report on Thursday, citing anonymous sources with insight into the PLA’s inner workings. The opposition is not the opinion of a few generals, but a widely shared view among the PLA cadre, the Epoch Times cited them as saying. “Chinese forces know full well that