A new cancer drug under development could begin phase 1 testing in humans next year after it obtained good results in treating eight types of cancers in animal tests, the National Health Research Institutes (NHRI) announced yesterday.
Hsieh Hsing-pang (謝興邦), a researcher at the NHRI’s Institute of Biotechnology and Pharmaceutical Research (IBPR), said at a media briefing that 40 late-stage cancer patients who have not been helped by any available treatment, but are still in decent physical condition, are to be recruited for phase 1 clinical trials next year.
The new drug, known as DBPR144, is a small molecule known as a multi-target kinase inhibitor, Hsieh said.

Photo: CNA
A kinase is an enzyme that controls important cell functions, and it can be active in the growth of some types of cancer cells.
The eight types of cancers against which it showed success through in vivo studies in animals were pancreatic, oral, gastric, liver, bladder, prostate and colorectal cancer, as well as acute myeloid leukemia, Hsieh said.
“Because it is particularly effective in gastroenterology-related cancers, we are likely to first target patients with pancreatic, liver, bladder and gastric cancer in the initial clinical trial,” he said.
Multi-target drugs, which take aim at several targets rather than more common medications that only target a single biological substance, more effectively inhibit cancer cell proliferation and overcome drug resistance, he said.
During the phase 1 trial, the volunteers are to be given DBPR114 once a week to determine safe dosages of the drug, Hsieh said.
Chen Chiu-heng (陳丘泓), general manager of Launxp Biomedical Co, which will be responsible for manufacturing the drug, said cancer patients from Taiwan and Australia are to be recruited to take part in the initial trial, which is expected to take two years.
Phase 2 and phase 3 trials, which should each take two to three years, will focus on cancers that responded promisingly to the drug in phase 1.
At least six years will be needed to complete human trials, Chen said.
“This novel, multi-targeted agent is our new hope for treating cancer patients,” IBPR Director Chang Jang-yang (張俊彥) said.
Cancer has been the top cause of death in Taiwan for 41 years, he said, and the five-year survival rates among patients with major types of cancer remained low, especially the 5 percent rate for people with pancreatic cancer.

Taiwan is to commence mass production of the Tien Kung (天弓, “Sky Bow”) III, IV and V missiles by the second quarter of this year if the legislature approves the government’s NT$1.25 trillion (US$39.78 billion) special defense budget, an official said yesterday. Commenting on condition of anonymity, a defense official with knowledge of the matter said that the advanced systems are expected to provide crucial capabilities against ballistic and cruise missiles for the proposed “T-Dome,” an advanced, multi-layered air defense network. The Tien Kung III is an air defense missile with a maximum interception altitude of 35km. The Tien Kung IV and V
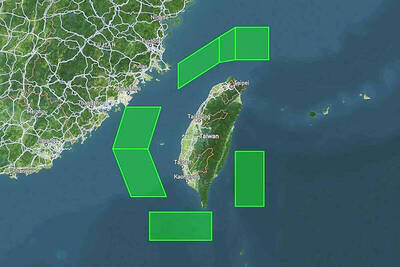
The disruption of 941 flights in and out of Taiwan due to China’s large-scale military exercises was no accident, but rather the result of a “quasi-blockade” used to simulate creating the air and sea routes needed for an amphibious landing, a military expert said. The disruptions occurred on Tuesday and lasted about 10 hours as China conducted live-fire drills in the Taiwan Strait. The Civil Aviation Administration (CAA) said the exercises affected 857 international flights and 84 domestic flights, affecting more than 100,000 travelers. Su Tzu-yun (蘇紫雲), a research fellow at the government-sponsored Institute for National Defense and Security Research, said the air

A strong continental cold air mass is to bring pollutants to Taiwan from tomorrow, the Ministry of Environment said today, as it issued an “orange” air quality alert for most of the country. All of Taiwan except for Hualien and Taitung counties is to be under an “orange” air quality alert tomorrow, indicating air quality that is unhealthy for sensitive groups. In China, areas from Shandong to Shanghai have been enveloped in haze since Saturday, the ministry said in a news release. Yesterday, hourly concentrations of PM2.5 in these areas ranged from 65 to 160 micrograms per cubic meter (mg/m³), and pollutants were

Taiwan’s armed forces have established response protocols for a wide range of sudden contingencies, including the “Wan Chun Plan” to protect the head of state, the Ministry of Defense (MND) said today. After US President Donald Trump on Saturday launched a series of airstrikes in Venezuela and kidnapped Venezuelan President Nicolas Maduro, concerns have been raised as to whether China would launch a similar “decapitation strike” on Taiwan. The armed forces regularly coordinate with relevant agencies and practice drills to ensure preparedness for a wide range of scenarios, Vice Minister of National Defense Hsu Szu-chien (徐斯儉) told reporters before a