The Taiwan Health Reform Foundation yesterday said over-the-counter (OTC) drugs should come with instructions that are easy to understand and drug facts should be printed on the package labeling.
Members of the foundation chanted slogans such as: “We want instructions, not textbooks,” and “Show us the warnings, don’t play hide and seek,” as they urged the Food and Drug Administration (FDA) to demand that drug companies improve their OTC drug labeling and instructions.
Foundation chairperson Joanne Liu (劉淑瓊) said the assembly of the first National Conference on Drug Policies in 2008 passed a resolution that said drug package inserts should be written more explicitly and in a way that makes them easier to understand, but six years later many drugs still come with instructions that are too difficult for most people to understand.
The foundation cited a definition written on the information insert of an OTC drug bought in Taiwan: “This product is a specialized and fast-effective histamine antagonist, which can inhibit the secretion of basal gastric acid and stimulated gastric acid. It can reduce secretion, reduce acidity and the amount of pepsin.”
Foundation chief executive officer Amy Wang (王梅影) said the information insert that came with the same drug bought in the US said the drug “reduces the production of stomach acid and is different from antacids, which neutralize the acid already in your stomach,” which is much easier to understand.
Wang said the drug facts printed on the package labeling of US-bought drugs follow a standard format, including the most important “caution” and a drug use consultation hotline, but the drug facts printed on the package labeling of Taiwan-bought drugs are printed on different sides of the box and are unclear.
Wang said the foundation asked two of its members to buy commonly used drugs — ibuprofen (often used as an anti-inflammatory) and diclofenac (often used in pain relief patches) — for their child and pregnant wife respectively.
They found that the drug labeling of one ibuprofen product said “please read the package insert for details” and did not mention that children under the age of 12 should not use the product, but the drug with the same ingredients from another brand had it clearly printed on the package label.
Liu said if the FDA did not revise its standards for reviewing drug product labeling and usage instructions, consumers might face the negative health risks of drug misuse.
The foundation said the FDA should provide a clear standard format of drug facts and package inserts for drug companies to follow.

The manufacture of the remaining 28 M1A2T Abrams tanks Taiwan purchased from the US has recently been completed, and they are expected to be delivered within the next one to two months, a source said yesterday. The Ministry of National Defense is arranging cargo ships to transport the tanks to Taiwan as soon as possible, said the source, who is familiar with the matter. The estimated arrival time ranges from late this month to early next month, the source said. The 28 Abrams tanks make up the third and final batch of a total of 108 tanks, valued at about NT$40.5 billion

Travel agencies in Taiwan are working to secure alternative flights for travelers bound for New Zealand for the Lunar New Year holiday, as Air New Zealand workers are set to strike next week. The airline said that it has confirmed that the planned industrial action by its international wide-body cabin crew would go ahead on Thursday and Friday next week. While the Auckland-based carrier pledged to take reasonable measures to mitigate the impact of the workers’ strike, an Air New Zealand flight arriving at Taipei from Auckland on Thursday and another flight departing from Taipei for Auckland on Saturday would have to
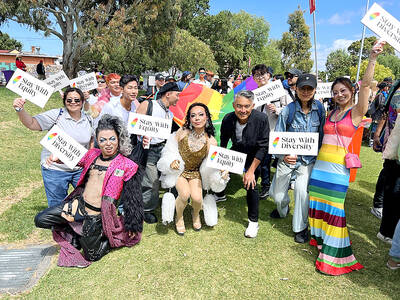
A group from the Taiwanese Designers in Australia association yesterday represented Taiwan at the Midsumma Pride March in Melbourne. The march, held in the St. Kilda suburb, is the city’s largest LGBTQIA+ parade and the flagship event of the annual Midsumma Festival. It attracted more than 45,000 spectators who supported the 400 groups and 10,000 marchers that participated this year, the association said. Taiwanese Designers said they organized a team to march for Taiwan this year, joining politicians, government agencies, professionals and community organizations in showing support for LGBTQIA+ people and diverse communities. As the first country in Asia to legalize same-sex

MOTIVES QUESTIONED The PLA considers Xi’s policies toward Taiwan to be driven by personal considerations rather than military assessment, the Epoch Times reports Chinese President Xi Jinping’s (習近平) latest purge of the Chinese People’s Liberation Army (PLA) leadership might have been prompted by the military’s opposition to plans of invading Taiwan, the Epoch Times said. The Chinese military opposes waging war against Taiwan by a large consensus, putting it at odds with Xi’s vision, the Falun Gong-affiliated daily said in a report on Thursday, citing anonymous sources with insight into the PLA’s inner workings. The opposition is not the opinion of a few generals, but a widely shared view among the PLA cadre, the Epoch Times cited them as saying. “Chinese forces know full well that