Following the discovery of maleic acid — an industrial chemical banned from use in the food industry — in products containing starch, the Consumers’ Foundation yesterday accused the Food and Drug Administration (FDA) of bureaucratic ineptitude and failure in its duty of supervision over food safety.
Two years to the month after a food scare in May 2011 over plasticizers being used in food additives, the FDA said on Monday that out of 74 randomly inspected food products, five were found to contain the banned organic compound.
The products found to be in violation of regulations included tapioca — which is widely used in bubble tea — and oden, which is sold in many convenience stores.
The foundation yesterday questioned why the FDA stopped short of revealing its findings immediately if, as the agency stated in a press release, it had received tip-offs about the use of industrial starch in mid-March and had discovered who was responsible a month later.
“The convenience store chain involved in the scandal was aware of the problem and pulled its oden products containing maleic acid off of its shelves on April 30, and the manufacturer said it stopped delivering these products from April 28 due to the contamination,” foundation chairman Mark Chang (張智剛) said.
“Despite these acts by the distributor and the producer, it took the FDA two weeks to announce the results of its tests,” Chang said, emphasizing that this means consumers had been unknowingly consuming maleic during this time.
“The foundation has been demanding the establishment of a food-traceability system, which was also mentioned during the plasticizer scandal, and the revision of related laws to rein in food manufacturers, but these calls have constantly been overlooked by the food authority,” Chang said.
Foundation board member Yu Kai-hsiung (游開雄) said the FDA had attempted to “conceal manufacturers’ guilt” by endorsing the harmlessness of consuming small amounts of the chemical.
At the height of the plasticizer scandal the FDA said on its Web site that 85 percent of any di(2-ethylhexyl) phthalate, or DEHP, consumed can be metabolized and excreted by the body within 72 hours.
“The agency again said of the present case that 30g of maleic acid a day is tolerable, adding that the chemical is neither carcinogenic nor genotoxic and does not pose risks to reproduction,” Chang said.
“They clearly do not take the health of the general public seriously,” Yu added.
“These food scandals have led the foundation to believe that the FDA has failed in its duty to supervise public food safety,” Chang said, who yesterday issued a complaint to the Control Yuan, calling for correction and censure of the FDA.
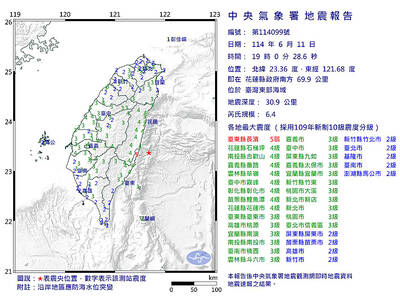
A magnitude 6.4 earthquake struck off the coast of Hualien County in eastern Taiwan at 7pm yesterday, the Central Weather Administration (CWA) said. The epicenter of the temblor was at sea, about 69.9km south of Hualien County Hall, at a depth of 30.9km, it said. There were no immediate reports of damage resulting from the quake. The earthquake’s intensity, which gauges the actual effect of a temblor, was highest in Taitung County’s Changbin Township (長濱), where it measured 5 on Taiwan’s seven-tier intensity scale. The quake also measured an intensity of 4 in Hualien, Nantou, Chiayi, Yunlin, Changhua and Miaoli counties, as well as

Credit departments of farmers’ and fishers’ associations blocked a total of more than NT$180 million (US$6.01 million) from being lost to scams last year, National Police Agency (NPA) data showed. The Agricultural Finance Agency (AFA) said last week that staff of farmers’ and fishers’ associations’ credit departments are required to implement fraud prevention measures when they serve clients at the counter. They would ask clients about personal financial management activities whenever they suspect there might be a fraud situation, and would immediately report the incident to local authorities, which would send police officers to the site to help, it said. NPA data showed

ENERGY RESILIENCE: Although Alaska is open for investments, Taiwan is sourcing its gas from the Middle East, and the sea routes carry risks, Ho Cheng-hui said US government officials’ high-profile reception of a Taiwanese representative at the Alaska Sustainable Energy Conference indicated the emergence of an Indo-Pacific energy resilience alliance, an academic said. Presidential Office Secretary-General Pan Men-an (潘孟安) attended the conference in Alaska on Thursday last week at the invitation of the US government. Pan visited oil and gas facilities with senior US officials, including US Secretary of the Interior Doug Burgum, US Secretary of Energy Chris Wright, Alaska Governor Mike Dunleavy and US Senator Daniel Sullivan. Pan attending the conference on behalf of President William Lai (賴清德) shows a significant elevation in diplomatic representation,

The Taipei City Reserve Command yesterday initiated its first-ever 14-day recall of some of the city’s civilian service reservists, who are to undergo additional training on top of refresher courses. The command said that it rented sites in Neihu District (內湖), including the Taipei Tennis Center, for the duration of the camp to optimize tactical positioning and accommodate the size of the battalion of reservists. A battalion is made up of four companies of more than 200 reservists each, it said. Aside from shooting drills at a range in New Taipei City’s Linkou District (林口), the remainder of the training would be at