Liberty Times: The Council of Agriculture (COA), after chairing the inter-departmental meeting, is alleged to have made an arbitrary statement that ractopamine-based Paylean differs from commonly known lean meat essences. Is Paylean not a kind of lean meat additive?
Lin Chieh-liang (林杰樑): Paylean is definitely a lean meat additive. The effects of the additive are exactly the same as that of other lean-meat promoting drugs, which are to increase lean meat in cattle and pigs and to decrease the amount of excrement to lower overhead costs and maximize economic value. Nobody in the entire world would deny that fact.
While low in toxicity in comparison with clenbuterol and salbutamol, Paylean cannot be said to be completely non-toxic. If [Paylean] is as safe as the government says it is, why is it that only 27 nations, and most of them exporters of farm animals, in the whole world dare use it? [The only explanation] is that its safety [of usage] is still doubted internationally.

Photo: Lo Pei-der, Taipei Times
Because Paylean causes animals to grow lean meat unnaturally, it must have adverse side-effects. It is possible that it would cause palpitations or induce side-effects including cardiovascular diseases. People with cardiovascular diseases do not even have to ingest a lot of it to be poisoned; [all it takes] is for their heartbeat to accelerate to higher than 100 beats per minute — from the normal 70 to 90 beats per minute — for symptoms to begin to show, or even become fatal.
Currently, 29 countries in the world have clear laws prohibiting the use of Paylean, while other nations, such as Singapore, do not allow the import or use of the additive. These countries have further said that they would only consider importing and using the additive if the WHO or the Codex Alimentarius Commission (Codex) has certified its safety.
LT: Premier Sean Chen (陳冲) and other high-level officials have all been saying that no research has ever showed that anyone has been poisoned by ingesting Paylean-laced meat. It seems that [they are implying] Paylean is safe.
Lin: The EU’s European Food Safety Authority (EFSA) released complete and in-depth scientific evaluation reports on Paylean in 2009.
These reports were based on documents and research reports that the US offered to the WHO on the use of Paylean in the US and it also included research — which the EFSA doubted very much — cited by our government officials, saying that six subject cases did not exhibit Paylean-poisoning symptoms.
The EFSA report said that the US’ data, with most of the statistics based on animal test subjects, had a credibility issue. It added that, for safety reasons, evaluations should also be done of human test subjects.
The EFSA also cast doubts on the US Food and Drug Administration’s (FDA) Acceptable Daily Intake (ADI) standard of 1 microgram of Paylean per person, based on a study of six test subjects that ingested 5 milligrams of Paylean each, saying that not enough people have been tested to form a basis for setting a standard.
The EFSA further said that of the six test subjects, one had backed out of the experiment after experiencing palpitations upon ingesting Paylean, showing the report lacked credibility.
The EFSA also questioned why the FDA had given the test subjects a lower dosage to determine Paylean’s effects, adding that all six subjects had clean bills of health. The FDA study did not even evaluate high-risk groups with cardiovascular diseases, the EFSA said, adding that the US report would not be able to predict the safety of high-risk groups.
Premier Chen and the COA minister kept emphasizing that there were no scientific reports of cases of Paylean poisoning. While they are certainly right, therein lies the problem — the lack of research into how Paylean affects the human body causes [public] worries.
Besides, there are a lot of high-risk groups who face risks to their health by the side-effects of drugs that speed up heartbeat rates.
The EU has since 2009 asked the US to provide more proof based on further tests on human subjects, especially on the epidemiology for high-risk groups, but because the US has so far not complied, the EU has kept Paylean on its prohibited list.
LT: The governmental health organizations had already in 2007 drafted an act tolerating a certain level of residual Paylean. Would such a measure ensure the safety of people ingesting US beef?
Lin: Neither the US’ suggestion of 1 microgram ADI, nor the Japanese standard of 10ppb (parts per billion) to 90ppb of residual Paylean in meats or intestines, correspond with the eating habits of Taiwanese.
The Americans focus mainly on the [consumption of] meat and the Japanese do not eat intestines, but Taiwanese eat almost everything. With the concentration of residual drugs higher in the intestines — at least five to ten times the amount found in meat — the danger [posed to people] is also that much higher.
Medical research in the past has by and large focused on the concentration of residuals in the livers and kidneys, but last year China announced research results according to which the lungs of cows also accumulate a high concentration of Paylean. This led China to tell the WHO that Paylean should be forbidden [in China], because the Chinese also eat the intestines and lungs of cows. This is the reason China forbids the importing of US beef.
In the same vein, Taiwanese also frequently eat intestines. For example, a lactating mother, in the month after giving birth, would almost on a daily basis eat kidneys and [intestines.]
According to an investigation by the Department of Health several years ago, pregnant women eat 360g of pig livers, 130g of pig kidneys and 92g of meat [during the month after giving birth], which would equal ingesting 27 micrograms of Paylean per day.
While these are average rates [gleaned from] old data and [taking into account that] there are those who do not eat meat at all, some people eat a lot of meat at one sitting. Clinically, I’ve seen cases where people ate at least 600g of meat every day.
Added to that, people now like to go to buffet-style barbeque restaurants, so it has become easy to eat too much meat and intestines every day.
The EU also said the risk evaluation and measuring of Paylean should not only be focused on the “actual Paylean concentration,” but the concentration of its metabolic intermediates, ie, its half-metabolized products, should also be measured.
Although the current examination does not test the metabolic intermediates like it does Paylean itself, the risk such amalgamates pose to the health of humans or animals is the same, and it should also be listed within the measurements [of potential harm to the human body].
Besides, feed additives for most pigs and cows on the final drive to the slaughterhouse are usually left out for a certain amount of time, so they would leave no residuals.
However, the use of Paylean is not stopped, even on the day of slaughter, and the chemical properties of Paylean are not damaged [or altered] even if the meat is cooked or boiled.
Part two of this interview will be published tomorrow.
Translated by Jake Chung, Staff Writer
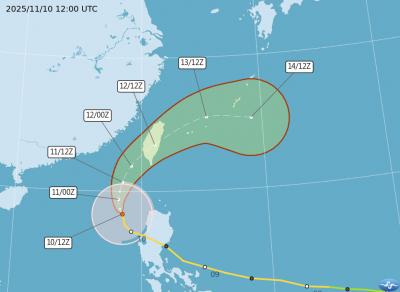
The Central Weather Administration (CWA) today issued a sea warning for Typhoon Fung-wong effective from 5:30pm, while local governments canceled school and work for tomorrow. A land warning is expected to be issued tomorrow morning before it is expected to make landfall on Wednesday, the agency said. Taoyuan, and well as Yilan, Hualien and Penghu counties canceled work and school for tomorrow, as well as mountainous district of Taipei and New Taipei City. For updated information on closures, please visit the Directorate-General of Personnel Administration Web site. As of 5pm today, Fung-wong was about 490km south-southwest of Oluanpi (鵝鑾鼻), Taiwan's southernmost point.
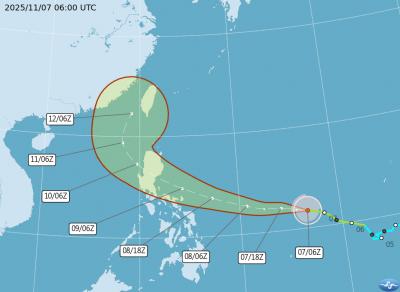
Tropical Storm Fung-Wong would likely strengthen into a typhoon later today as it continues moving westward across the Pacific before heading in Taiwan’s direction next week, the Central Weather Administration (CWA) said. As of 8am, Fung-Wong was about 2,190km east-southeast of Cape Oluanpi (鵝鑾鼻), Taiwan’s southernmost point, moving westward at 25kph and possibly accelerating to 31kph, CWA data showed. The tropical storm is currently over waters east of the Philippines and still far from Taiwan, CWA forecaster Tseng Chao-cheng (曾昭誠) said, adding that it could likely strengthen into a typhoon later in the day. It is forecast to reach the South China Sea

Almost a quarter of volunteer soldiers who signed up from 2021 to last year have sought early discharge, the Legislative Yuan’s Budget Center said in a report. The report said that 12,884 of 52,674 people who volunteered in the period had sought an early exit from the military, returning NT$895.96 million (US$28.86 million) to the government. In 2021, there was a 105.34 percent rise in the volunteer recruitment rate, but the number has steadily declined since then, missing recruitment targets, the Chinese-language United Daily News said, citing the report. In 2021, only 521 volunteers dropped out of the military, the report said, citing

Nearly 5 million people have signed up to receive the government’s NT$10,000 (US$322) universal cash handout since registration opened on Wednesday last week, with deposits expected to begin tomorrow, the Ministry of Finance said yesterday. After a staggered sign-up last week — based on the final digit of the applicant’s national ID or Alien Resident Certificate number — online registration is open to all eligible Taiwanese nationals, foreign permanent residents and spouses of Taiwanese nationals. Banks are expected to start issuing deposits from 6pm today, the ministry said. Those who completed registration by yesterday are expected to receive their NT$10,000 tomorrow, National Treasury