Taiwan plans to take steps to force the Swiss pharmaceutical company Roche to let the country produce the anti-bird flu drug Tamiflu, the health minister said yesterday.
The move is part of a "two-track" approach for obtaining a Tamiflu license so Taiwan can combat a possible bird-flu outbreak, Department of Health Minister Hou Sheng-mou (侯勝茂) said.
Hou said his ministry has asked the bureau in charge of intellectual property rights to request a "coercive authorization" for the drug's production, citing a national emergency clause.
"The Department of Health, while seeking approval from Roche, has asked the Economic Ministry's Intellectual Property Office [IPO] for compulsory licensing to produce Tamiflu," Hou told the Legislative Yuan.
"The license could be granted by the end of November at the earliest," he said.
"The production will boost our stockpile of Tamiflu to enough for 3 million people," Hou said.
The bureau is expected to contact Roche to learn its position on the issue before making a judgment in about a month, he said.
WTO regulations allow for drug patents to be violated in the event of medical emergencies, providing that the patent holder is compensated at a later date.
Taiwan submitted an application to Roche on Oct. 17 for a sublicense to produce its version of Tamiflu. Roche said later that it was willing to discuss details with Taipei.
The two sides are scheduled to hold their first talks on Nov. 8.
Roche has said it is willing to negotiate on Tamiflu manufacture with countries or companies able to produce large amounts of the drug if they meet appropriate quality specifications, safety and regulatory guidelines.
The government recently said it has succeeded in developing a generic version, which it said is 99 percent akin to Tamiflu, and has bought 3 tonnes of shikimic acid, which is the raw material for making Tamilfu and is enough to make the drug for 2.3 million people -- about 10 percent of the country's population.
Premier Frank Hsieh (
The next step in Taiwan's efforts to produce its own version of the drug, which doesn't cure bird flu but can ease and shorten its symptoms, is in the IPO's hands. Upon receiving the application for compulsory licensing, the office will send a duplicate copy of the the application to Roche to request a response.
If no response is given, the office will grant the compulsory license.
An IPO official told reporters that the standard time for the patent holder to respond is three months.
"But in a case of emergency, it could be a few days or even one day," the official said.
As for the amount of compensation, "it is up to the applicant and the patent holder to discuss," the official in the IPO's legal affairs department said. "If they have failed to reach an agreement, IPO will make a ruling."

The Coast Guard Administration (CGA) yesterday said it had deployed patrol vessels to expel a China Coast Guard ship and a Chinese fishing boat near Pratas Island (Dongsha Island, 東沙群島) in the South China Sea. The China Coast Guard vessel was 28 nautical miles (52km) northeast of Pratas at 6:15am on Thursday, approaching the island’s restricted waters, which extend 24 nautical miles from its shoreline, the CGA’s Dongsha-Nansha Branch said in a statement. The Tainan, a 2,000-tonne cutter, was deployed by the CGA to shadow the Chinese ship, which left the area at 2:39pm on Friday, the statement said. At 6:31pm on Friday,
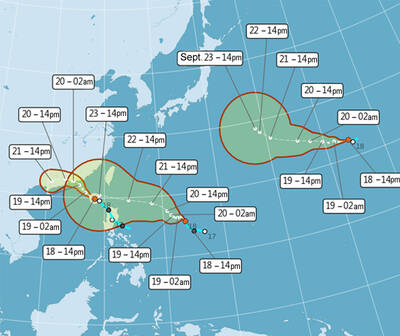
PEAK MONTHS: Data showed that on average 25 to 27 typhoons formed in the Pacific and South China seas annually, with about four forming per month in July and October One of three tropical depressions in the Pacific strengthened into a typhoon yesterday afternoon, while two others are expected to become typhoons by today, Central Weather Administration (CWA) forecaster Lee Ming-hsiang (李名翔) said yesterday. The outer circulation of Tropical Depression No. 20, now Typhoon Mitag, has brought light rain to Hualien, Taitung and areas in the south, Lee said, adding that as of 2pm yesterday, Mitag was moving west-northwest at 16kph, but is not expected to directly affect Taiwan. It was possible that Tropical Depression No. 21 would become a typhoon as soon as last night, he said. It was moving in a

The American Institute in Taiwan (AIT) put Taiwan in danger, Ma Ying-jeou Foundation director Hsiao Hsu-tsen (蕭旭岑) said yesterday, hours after the de facto US embassy said that Beijing had misinterpreted World War II-era documents to isolate Taiwan. The AIT’s comments harmed the Republic of China’s (ROC) national interests and contradicted a part of the “six assurances” stipulating that the US would not change its official position on Taiwan’s sovereignty, Hsiao said. The “six assurances,” which were given by then-US president Ronald Reagan to Taiwan in 1982, say that Washington would not set a date for ending arm sales to Taiwan, consult

A Taiwanese academic yesterday said that Chinese Ambassador to Denmark Wang Xuefeng (王雪峰) disrespected Denmark and Japan when he earlier this year allegedly asked Japan’s embassy to make Taiwan’s representatives leave an event in Copenhagen. The Danish-language Berlingske on Sunday reported the incident in an article with the headline “The emperor’s birthday ended in drama in Copenhagen: More conflict may be on the way between Denmark and China.” It said that on Feb. 26, the Japanese embassy in Denmark held an event for Japanese Emperor Naruhito’s birthday, with about 200 guests in attendance, including representatives from Taiwan. After addressing the Japanese hosts, Wang