The inspection of the first four batches of the domestic COVID-19 vaccine has been completed and the doses are ready to be rolled out, the Food and Drug Administration (FDA) said yesterday.
The inspection of the four batches of the vaccine made by Medigen Vaccine Biologics Corp (高端疫苗), a total of 265,528 doses, was completed on Friday last week and they are being sealed at a designated warehouse in preparation for use, FDA Research and Inspection Division head Wang Teh-yuan (王德原) said.
The sealing was expected to be completed yesterday evening, he said.
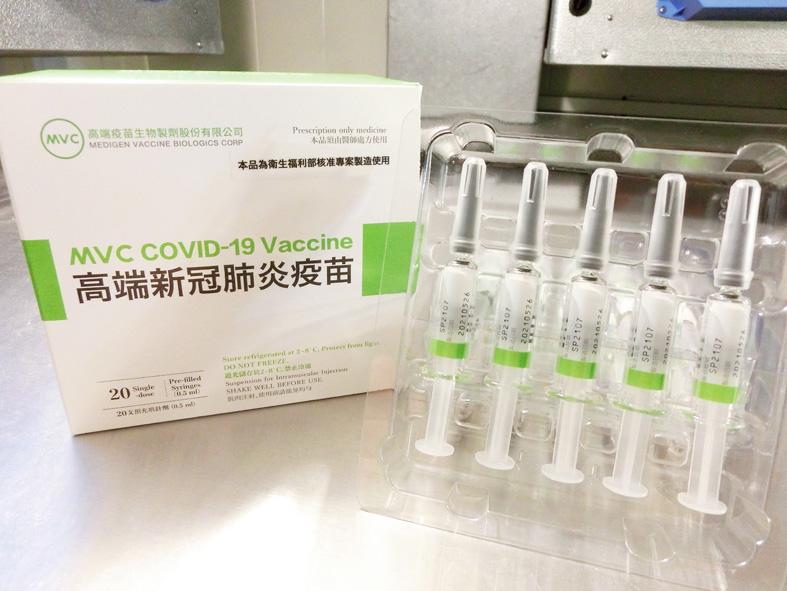
Photo courtesy of the Food and Drug Administration
The Medigen vaccine, the first protein-based COVID-19 vaccine to be made available in Taiwan, would be good for six months before they expire, he added.
Medigen secured an emergency use authorization (EUA) for the vaccine from the FDA on July 19.
It took the FDA about one month to carry out the inspection of the first four batches of the protein-based vaccine, which is different from the spike-protein-based AstraZeneca vaccine and the mRNA-based Moderna vaccine previously received from overseas, Wang said, adding that the inspection took longer than the other two vaccines, including 21 days in animal testing to ensure its efficacy.
The Central Epidemic Command Center said that the Medigen vaccine would be offered in the national vaccination program when there is a sufficient number of doses.
Minister of Health and Welfare Chen Shih-chung (陳時中), who heads the center, said it would be difficult to distribute the vaccine when there are so few doses available, adding that the center would wait until about 500,000 to 600,000 doses have passed testing before offering it in the program.
Asked whether the Medigen vaccine went into mass production and lot release testing before it received an EUA, Chen said that the EUA would not have been issued just because the product had gone into mass production, adding that when facing a pandemic, some vaccine development steps were conducted in parallel without increasing the risk for study participants to accelerate its development.
The center had requested that the Medigen vaccine go into mass production prior to the EUA review, Chen said, adding that according to the procurement agreement signed with the company, if the vaccine had failed to obtain an EUA then production costs would have been paid by the center.
Asked why the lot numbers of the four batches that passed testing were not sequential, Chen said that some of the batches still have problems that need to be solved, such as lacking the required technical information for review.
Meanwhile, Chen said that as the number of doses of the AstraZeneca vaccine is running short, the center plans to offer doses of the Moderna vaccine in the next round of bookings.
After 140,000 Moderna doses had been reserved for the second jabs of people in the top three priority groups, pregnant women and people aged 65 or older, there would be about 420,000 doses offered to other people who have registered on the national vaccination booking system, he said.
Eligible recipients of the Moderna vaccine in the next round would mainly be people in the ninth priority group of people who have a high-risk disease, a rare disease or catastrophic illness, he added.
There are about 880,000 people in the ninth priority group who only selected the Moderna vaccine, Chen said.

An Emirates flight from Dubai arrived at Taiwan Taoyuan International Airport yesterday afternoon, the first service of the airline since the US and Israel launched strikes against Iran on Saturday. Flight EK366 took off from the United Arab Emirates (UAE) at 3:51am yesterday and landed at 4:02pm before taxiing to the airport’s D6 gate at Terminal 2 at 4:08pm, data from the airport and FlightAware, a global flight tracking site, showed. Of the 501 passengers on the flight, 275 were Taiwanese, including 96 group tour travelers, the data showed. Tourism Administration Deputy Director-General Huang He-ting (黃荷婷) greeted Taiwanese passengers at the airport and

POSSIBILITIES EMERGE: With Taiwan’s victory and Japan’s narrow win over Australia, Taiwan now have a chance to advance if South Korea also beat the Aussies Taiwan has high hopes that the national baseball team would advance to the World Baseball Classic (WBC) quarter-finals after clinching a crucial 5-4 victory over South Korea in a nail-biting extra-inning game at the Tokyo Dome yesterday. Boosted by three home runs — two solo shots by Yu Chang (張育成) and Cheng Tsung-che (鄭宗哲) and a two-run homer by Stuart Fairchild — the triumph gave Taiwan a much-needed second victory in the five-team Pool C, where only the top two finishers would advance to the knockout stage in Miami, Florida. Entering extra innings with the game tied at four apiece, Taiwan scored

STRAIT OF HORMUZ: In the case of a prolonged blockade by Iran, Taiwan would look to sources of LNG outside the Middle East, including Australia and the US Taiwan would not have to ration power due to a shortage of natural gas, Minister of Economic Affairs Kung Ming-hsin (龔明鑫) said yesterday, after reports that the Strait of Hormuz was closed amid the conflict in the Middle East. The government has secured liquefied natural gas (LNG) supplies for this month and contingency measures are in place if the conflict extends into next month, Kung told lawmakers. Saying that 25 percent of Taiwan’s natural gas supplies are from Qatar, Chinese Nationalist Party (KMT) caucus secretary-general Lin Pei-hsiang (林沛祥) asked about the situation in light of the conflict. There would be “no problems” with

PLANE HIT: The Israeli military said it shot down an Iranian Air Force fighter over Tehran, while an Iranian warship sank off Sri Lanka, with no cause known The US and Israel yesterday hit Iran’s capital and other cities in multiple airstrikes on the fifth day of the war with Iran. Israel targeted the Iranian leadership and security forces, while the Islamic Republic responded with missile barrages and drone attacks on Israel, and across the region. Tehran residents woke to dawn blasts and Iranian state television showed the ruins of building in the center of the capital. The Shiite seminary city of Qom and multiple other cities were also targeted. With fighter jets roaring overhead, those still in Tehran looked anxiously to the skies. One man, who ran a clothing shop,