Concern over COVID-19 stems not only from its high degree of virulence, but from its ability to continuously mutate and produce new variants. An effective vaccine can help slow the virus’ spread, but the question of how to deal with its continuous mutations is key.
Mass production of the most well-known novel coronavirus vaccines in production, including by AstraZeneca PLC, Moderna Inc and Pfizer Inc, has a high technological threshold, especially for those using messenger RNA (mRNA) technology, since manufacturers need to overcome the challenges of storage and transportation at ultra-low temperatures.
Founded in 2018, Ascendo Biotechnology Inc (先知生技) has a research and development team with 30 years of clinical drug research experience, focused on immune-related drug research.

Photo courtesy of Ascendo Biotechnology Inc
Using quantum mechanics technologies, Ascendo has developed a proprietary NanoCherubTM electric-kinetic nanocomplex antigen adjuvant and delivery platform, designed to rapidly respond to mutations without the challenges of ultra-low temperature storage and transportation, and to produce large quantities of vaccines quickly
‧ NanoCherubTM in the fight against COVID-19
Doctors and scientists have been racking their brains for a solution to the constantly mutating virus. Using quantum mechanics calculations, Ascendo can produce effective vaccines quickly, with a precise and flexible technology circumventing the need for the constant testing of more traditional approaches.
Last year, Ascendo started working with academic research institutions in Taiwan on developing a COVID-19 vaccine, for the first time using quantum mechanics technology to design a drug delivery system encapsulating antigens to form a positively charged nanocomplex, enabling immune cells to generate a strong and effective response to the NanoCherubTM Electric-Kinetic nanocomplex and achieving a balanced and robust Th1/Th2 immunity.
The ASD254 vaccine developed by Ascendo last year showed excellent results in animal studies using mice, which most closely replicate the symptoms of the novel coronavirus in humans.
All vaccinated animals received 100 percent immune protection when tested with the live virus. Ascendo soon plans to apply to the Food and Drug Administration for phase 1 human trials, hoping to enter mass production next year.
The drug delivery platform can significantly shorten development times and respond to virus mutations, and the NanoCherubTM Electric-Kinetic nanocomplex can consistently induce balanced and strong Th1/Th2 immunity against encapsulated antigens, including full pathogens, subunit proteins, peptides and messenger ribonucleic acid, with the added advantage of the vaccines only needing to be stored at 2°C to 8°C.
‧ Hepatitis B therapeutic vaccine completed in animal efficacy trials
Ascendo has also completed animal efficacy trials for its ASD253 vaccine for chronic hepatitis B. Following preclinical trials, Phase 1 human trials are to begin next year, when ASD253 is expected to become the world’s first vaccine for chronic hepatitis B.
The company also hopes to develop vaccines for HIV (AIDS), dengue fever, Zika and pan-influenza viruses.
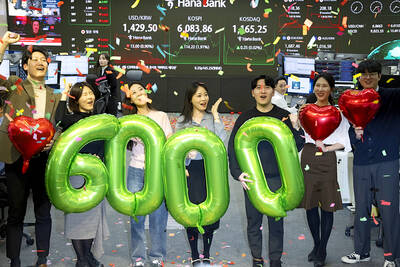
South Korea’s equity benchmark yesterday crossed a new milestone just a month after surpassing the once-unthinkable 5,000 mark as surging global memory demand powers the country’s biggest chipmakers. The KOSPI advanced as much as 2.6 percent to a record 6,123, with Samsung Electronics Co and SK Hynix Inc each gaining more than 2 percent. With the benchmark now up 45 percent this year, South Korea’s stock market capitalization has also moved past France’s, following last month’s overtaking of Germany’s. Long overlooked by foreign funds, despite being undervalued, South Korean stocks have now emerged as clear winners in the global market. The so-called “artificial intelligence

Chinese artificial intelligence (AI) start-up DeepSeek’s (深度求索) latest AI model, set to be released as soon as next week, was trained on Nvidia Corp’s most advanced AI chip, the Blackwell, a senior official of US President Donald Trump’s administration said on Monday, in what could represent a violation of US export controls. The US believes DeepSeek will remove the technical indicators that might reveal its use of American AI chips, the official said, adding that the Blackwells are likely clustered at its data center in Inner Mongolia, an autonomous region of China. The person declined to say how the US government received

FORTUNES REVERSED: The new 15 percent levies left countries with a 10 percent tariff worse off and stripped away the advantage of those with a 15 percent rate In a swift reversal of fortunes, countries that had been hardest hit by US President Donald Trump’s tariffs have emerged as the biggest winners from the US Supreme Court’s decision to strike down his emergency levies. China, India and Brazil are among those now seeing lower tariff rates for shipments to the US after the court ruled Trump’s use of the International Emergency Economic Powers Act to impose duties was illegal. While Trump subsequently announced plans for a 15 percent global rate, Bloomberg Economics said that would mean an average effective tariff rate of about 12 percent — the lowest since

Standard Chartered Bank Taiwan’s newly appointed chief executive officer, Anthony Yu (游天立), yesterday unveiled an ambitious growth strategy for the bank’s wealth management division, reflecting a bullish outlook on Taiwan’s high-net-worth market. Yu, the first local executive to lead Standard Chartered Bank’s Taiwan operations, emphasized rising client demand and detailed plans to expand the bank’s digital capabilities, as well as its physical presence across the country. Standard Chartered Taiwan saw a remarkable surge in new wealth management clients last month, with the number of clients holding assets equivalent to US$1 million more than doubling compared with the same month last year, he