Shire PLC plans to resubmit its application for US approval of a potential blockbuster drug for dry-eye disease next year after regulators unexpectedly asked for additional testing and information.
“We were disappointed” by the feedback on the medicine, called lifitegrast, CEO Flemming Ornskov said in a statement, adding that the Dublin-based drugmaker would soon get data from an advanced study called Opus-3 that might help with a resubmission to the US Food and Drug Administration (FDA).
Lifitegrast is one of the medicines in development that Shire is counting on to meet a goal of boosting sales to US$10 billion by 2020 from about US$6 billion last year.
Annual revenue from the drug could reach US$723 million by that date, according to the average of analyst estimates compiled by Bloomberg.
The company said late on Friday that the FDA had requested another clinical study and data on product quality, a setback after the drug had won priority review status, shortening the review process to eight months from the standard 12 months.
“Opus-3 has now been completed and top-line data are expected before the end of the year,” Ornskov said in the statement yesterday. “If the study is positive, we plan to refile our lifitegrast submission in the first quarter of 2016, and will remain on track for the planned launch next year.”
Opus-3 is “very likely” to show that it improves symptoms in patients, said David Evans, an analyst at UBS Group AG in London.
This would add evidence to two previous studies, neither of which hit both primary goals, he said.
In a research note earlier this month, Evans correctly predicted the FDA would want more data for the drug, which may at its peak hit US$2 billion in annual sales. He also said he expects it to eventually reach the market.
“We see a high probability of refiling and ultimate approval,” Evans said in the note.
Shire said that in the US there are 29 million adults who have the symptoms of dry-eye disease. The condition makes it more difficult to perform certain activities, including using a computer or reading for an extended period of time. Lifitegrast, if approved, would compete with Allergan PLC’s Restasis, which is estimated to bring in more than US$1 billion in sales this year.

Intel Corp chief executive officer Lip-Bu Tan (陳立武) is expected to meet with Taiwanese suppliers next month in conjunction with the opening of the Computex Taipei trade show, supply chain sources said on Monday. The visit, the first for Tan to Taiwan since assuming his new post last month, would be aimed at enhancing Intel’s ties with suppliers in Taiwan as he attempts to help turn around the struggling US chipmaker, the sources said. Tan is to hold a banquet to celebrate Intel’s 40-year presence in Taiwan before Computex opens on May 20 and invite dozens of Taiwanese suppliers to exchange views
Application-specific integrated circuit designer Faraday Technology Corp (智原) yesterday said that although revenue this quarter would decline 30 percent from last quarter, it retained its full-year forecast of revenue growth of 100 percent. The company attributed the quarterly drop to a slowdown in customers’ production of chips using Faraday’s advanced packaging technology. The company is still confident about its revenue growth this year, given its strong “design-win” — or the projects it won to help customers design their chips, Faraday president Steve Wang (王國雍) told an online earnings conference. “The design-win this year is better than we expected. We believe we will win

Chizuko Kimura has become the first female sushi chef in the world to win a Michelin star, fulfilling a promise she made to her dying husband to continue his legacy. The 54-year-old Japanese chef regained the Michelin star her late husband, Shunei Kimura, won three years ago for their Sushi Shunei restaurant in Paris. For Shunei Kimura, the star was a dream come true. However, the joy was short-lived. He died from cancer just three months later in June 2022. He was 65. The following year, the restaurant in the heart of Montmartre lost its star rating. Chizuko Kimura insisted that the new star is still down
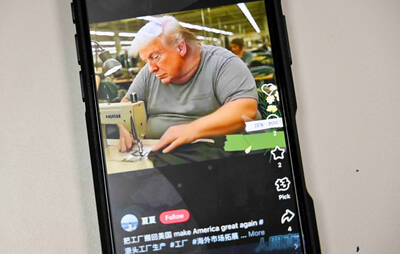
While China’s leaders use their economic and political might to fight US President Donald Trump’s trade war “to the end,” its army of social media soldiers are embarking on a more humorous campaign online. Trump’s tariff blitz has seen Washington and Beijing impose eye-watering duties on imports from the other, fanning a standoff between the economic superpowers that has sparked global recession fears and sent markets into a tailspin. Trump says his policy is a response to years of being “ripped off” by other countries and aims to bring manufacturing to the US, forcing companies to employ US workers. However, China’s online warriors