The US Food and Drug Administration (FDA) has approved the second human trial of human embryonic stem cells — this one testing cells in people with a progressive form of blindness, the company said yesterday.
Worcester, Massachusetts-based Advanced Cell Technology (ACT) said it would start testing its stem cell-based treatment on 12 patients with Stargardt’s macular dystrophy.
SECOND APPROVAL
It is the second trial of human embryonic stem cells to be approved by the FDA this year.
Last month, Geron Corp enrolled the first patient in its study using the cells in people whose spinal cords have been crushed.
GAMBLE
“It is exciting — a vindication. All this work really came through,” said Robert Lanza, chief medical officer of the company, which has struggled to stay solvent as it gambled on the controversial cells.
Stem cells are the body’s master cells, the source of all other cells.
Proponents of using embryonic stem cells say the field could transform medicine, providing treatments for brain diseases like Parkinson’s, juvenile diabetes or severe injuries.
However, opponents object because to get the cells, someone has to take apart a human embryo.
CHANGE
Last year, the administration of US President Barack Obama overturned the strictest of the limitations on using federal funds for the research, but this summer, two researchers challenged the policy.
A US appeals court has ruled that funding could continue while the government appeals, but grants from the US National Institutes of Health have been frozen and unfrozen as various courts have weighed in.
STARGARDT’S DISEASE
Stargardt’s disease causes progressive vision loss, usually starting in children or young adults 10 to 20 years old, as eye tissue called the retinal pigment epithelium, or RPE, degenerates.
There is currently no treatment for Stargardt’s disease.
ACT has coaxed human embryonic stem cells into becoming RPE cells, which will be infused into patients’ eyes.
BOUNTIFUL SUPPLY
“We can generate a virtually unlimited supply of healthy RPE cells,” Lanza said.
The company uses a unique type of human embryonic stem cell, taken from embryos left over at fertility clinics.
A single cell is removed when the embryo only has about eight cells — a process sometimes used when clinics want to test the embryos of people with genetic diseases to ensure that they are healthy.
CONTROVERSY
In theory, the embryo can continue developing, but in this case ACT has not implanted the embryo.
In the past, the company hoped this method of generating human embryonic stem cells would be less controversial than using an entire embryo.
Lanza said getting FDA approval for the trial was difficult.
“They had us jumping through hoops,” he said.
This was in part because the powerful embryonic cells have the power to give rise to all cell types and can cause teratomas — strange tumors containing a mix of cells.
ONE CELL ONLY
Lanza said the company can find even a single cell that might do this and remove it from the treatment batch.
In addition, he said treating an eye disease offers the opportunity to watch the treatment.
“We can look into the eye in real time and see what is going on,” he said.
APPROVAL PENDING
Research centers at the University of Oregon and University of Massachusetts will begin enrolling patients after their internal Institutional Review Boards approve the trial.
“My guess is that we could start in as soon as two to three months,” Lanza said.

Real estate agent and property developer JSL Construction & Development Co (愛山林) led the average compensation rankings among companies listed on the Taiwan Stock Exchange (TWSE) last year, while contract chipmaker Taiwan Semiconductor Manufacturing Co (TSMC, 台積電) finished 14th. JSL Construction paid its employees total average compensation of NT$4.78 million (US$159,701), down 13.5 percent from a year earlier, but still ahead of the most profitable listed tech giants, including TSMC, TWSE data showed. Last year, the average compensation (which includes salary, overtime, bonuses and allowances) paid by TSMC rose 21.6 percent to reach about NT$3.33 million, lifting its ranking by 10 notches

SEASONAL WEAKNESS: The combined revenue of the top 10 foundries fell 5.4%, but rush orders and China’s subsidies partially offset slowing demand Taiwan Semiconductor Manufacturing Co (TSMC, 台積電) further solidified its dominance in the global wafer foundry business in the first quarter of this year, remaining far ahead of its closest rival, Samsung Electronics Co, TrendForce Corp (集邦科技) said yesterday. TSMC posted US$25.52 billion in sales in the January-to-March period, down 5 percent from the previous quarter, but its market share rose from 67.1 percent the previous quarter to 67.6 percent, TrendForce said in a report. While smartphone-related wafer shipments declined in the first quarter due to seasonal factors, solid demand for artificial intelligence (AI) and high-performance computing (HPC) devices and urgent TV-related orders
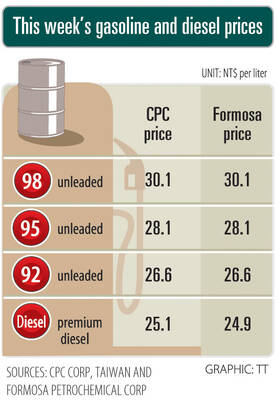
Prices of gasoline and diesel products at domestic fuel stations are this week to rise NT$0.2 and NT$0.3 per liter respectively, after international crude oil prices increased last week, CPC Corp, Taiwan (台灣中油) and Formosa Petrochemical Corp (台塑石化) said yesterday. International crude oil prices last week snapped a two-week losing streak as the geopolitical situation between Russia and Ukraine turned increasingly tense, CPC said in a statement. News that some oil production facilities in Alberta, Canada, were shut down due to wildfires and that US-Iran nuclear talks made no progress also helped push oil prices to a significant weekly gain, Formosa said

MINERAL DIPLOMACY: The Chinese commerce ministry said it approved applications for the export of rare earths in a move that could help ease US-China trade tensions Chinese Vice Premier He Lifeng (何立峰) is today to meet a US delegation for talks in the UK, Beijing announced on Saturday amid a fragile truce in the trade dispute between the two powers. He is to visit the UK from yesterday to Friday at the invitation of the British government, the Chinese Ministry of Foreign Affairs said in a statement. He and US representatives are to cochair the first meeting of the US-China economic and trade consultation mechanism, it said. US President Donald Trump on Friday announced that a new round of trade talks with China would start in London beginning today,