Thirteen pharmaceutical companies in Taiwan have been ordered to recall products and are facing fines for using food-grade ingredients instead of pharmaceutical-grade ingredients in stomach medicines, the Food and Drug Administration (FDA) said yesterday.
In an inspection of 27 pharmaceutical firms over the past three days, 13 could not prove that magnesium carbonate or calcium carbonate used in a total of 23 products were certified pharmaceutical ingredients, the agency said.
When magnesium carbonate and calcium carbonate are used as the major ingredients in pharmaceutical products, they must be of pharmaceutical grade, which requires a certificate from their suppliers, the agency said.
It said the 13 companies were using food-grade compounds as the major ingredients in their stomach medicines, in violation of FDA regulations.
FDA Director-General Chiang Yu-mei (姜郁美) said the 13 companies have been given an order to recall all of the problematic products within 30 days, but must ensure that they are removed from store shelves by midnight on Wednesday.
The agency also issued an order to seal the companies’ inventories and suspend their shipments, Chiang said.
Under the Pharmaceutical Affairs Act (藥事法), the companies are to be fined between NT$60,000 and NT$300,000, the agency said.
Earlier this month, the agency began inspecting pharmaceutical companies in the wake of reports that some of them were using magnesium carbonate and calcium carbonate intended for industrial use only.
Magnesium carbonate is widely marketed as a health supplement and is also used to make medicines for stomach ailments.
Its active ingredient, magnesium, is a mineral required for efficient functioning of the body.

“China is preparing to invade Taiwan,” Deputy Minister of Foreign Affairs Francois Wu (吳志中) said in an exclusive interview with British media channel Sky News for a special report titled, “Is Taiwan ready for a Chinese invasion?” the Ministry of Foreign Affairs said today in a statement. The 25-minute-long special report by Helen Ann-Smith released yesterday saw Sky News travel to Penghu, Taoyuan and Taipei to discuss the possibility of a Chinese invasion and how Taiwan is preparing for an attack. The film observed emergency response drills, interviewed baseball fans at the Taipei Dome on their views of US President
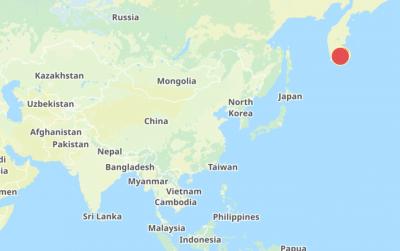
The Central Weather Administration (CWA) today issued a "tsunami watch" alert after a magnitude 8.7 earthquake struck off the Kamchatka Peninsula in northeastern Russia earlier in the morning. The quake struck off the east coast of the Kamchatka Peninsula at 7:25am (Taiwan time) at a depth of about 19km, the CWA said, citing figures from the Pacific Tsunami Warning Center. The CWA's Seismological Center said preliminary assessments indicate that a tsunami could reach Taiwan's coastal areas by 1:18pm today. The CWA urged residents along the coast to stay alert and take necessary precautions as waves as high as 1m could hit the southeastern

The National Museum of Taiwan Literature is next month to hold an exhibition in Osaka, Japan, showcasing the rich and unique history of Taiwanese folklore and literature. The exhibition, which is to run from Aug. 10 to Aug. 20 at the city’s Central Public Hall, is part of the “We Taiwan” at Expo 2025 series, highlighting Taiwan’s cultural ties with the international community, National Museum of Taiwan Literature director Chen Ying-fang (陳瑩芳) said. Folklore and literature, among Taiwan’s richest cultural heritages, naturally deserve a central place in the global dialogue, Chen said. Taiwan’s folklore would be immediately apparent at the entrance of the

ECONOMIC BENEFITS: The imports from Belize would replace those from Honduras, whose shrimp exports have dropped 67 percent since cutting ties in 2023 Maintaining ties with Taiwan has economic benefits, Ministry of Foreign Affairs officials said yesterday, citing the approval of frozen whiteleg shrimp imports from Belize by the Food and Drug Administration (FDA) as an example. The FDA on Wednesday approved the tariff-free imports from Belize after the whiteleg shrimp passed the Systematic Inspection of Imported Food, which would continue to boost mutual trade, the ministry said. Taiwan’s annual consumption of whiteleg shrimps stands at 30,000 tonnes, far exceeding domestic production, the ministry said. Taiwan used to fill the gap by importing shrimps from Honduras, but purchases slumped after Tegucigalpa severed diplomatic ties with Taiwan