British drugmaker GlaxoSmithKline (GSK) is to seek marketing approval for the world’s first malaria vaccine next year after trial data showed the shot significantly cut cases of the disease in African children.
The vaccine known as RTS,S was found, after 18 months of follow-up, to have almost halved the number of malaria cases in young children in the trial, and to have reduced by about a quarter the number of malaria cases in infants.
“Based on these data, GSK now intends to submit, in 2014, a regulatory application to the European Medicines Agency [EMA],” GSK, which has been developing the vaccine for three decades, said in a statement.
It added that the UN health agency, the Geneva-based WHO, has indicated it may recommend use of the RTS,S vaccine from as early as 2015 if EMA drugs regulators back its license application.
Malaria, a mosquito-borne parasitic disease, kills hundreds of thousands of people a year, mainly babies in the poorest parts of sub-Saharan Africa, and scientists say an effective vaccine is key to attempts to eradicate it.
Yet hopes that RTS,S would be the final answer were dampened last year when results from a final-stage trial with 6,537 babies aged six to 12 weeks showed the shot provided only modest protection, reducing episodes of the disease by 30 percent, compared with immunization with a control vaccine.
The latest readout on Tuesday last week from the malaria trial, which is Africa’s largest-ever clinical trial involving almost 15,500 children in seven countries, were presented at a medical meeting in Durban, South Africa.
GSK is developing RTS,S with the non-profit PATH Malaria Vaccine Initiative (MVI), with grant funding from the Bill & Melinda Gates Foundation to MVI.
“Many millions of malaria cases fill the wards of our hospitals. Progress is being made with bed nets and other measures, but we need more tools to battle this terrible disease,” said Halidou Tinto, a lead investigator on the RTS,S trial from Burkina Faso.
Previous data sets released from earlier parts of the trial showed the vaccine’s efficacy was 65 percent in babies analyzed six months after vaccination and only about 50 percent in five-month to 17-month-olds.
Nearly half of China’s major cities are suffering “moderate to severe” levels of subsidence, putting millions of people at risk of flooding, especially as sea levels rise, according to a study of nationwide satellite data released yesterday. The authors of the paper, published by the journal Science, found that 45 percent of China’s urban land was sinking faster than 3mm per year, with 16 percent at more than 10mm per year, driven not only by declining water tables, but also the sheer weight of the built environment. With China’s urban population already in excess of 900 million people, “even a small portion
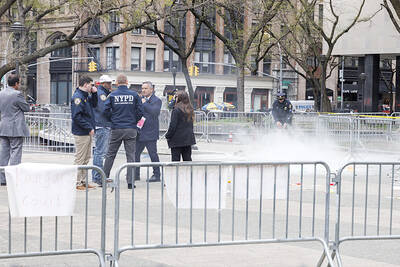
UNSETTLING IMAGES: The scene took place in front of TV crews covering the Trump trial, with a CNN anchor calling it an ‘emotional and unbelievably disturbing moment’ A man who doused himself in an accelerant and set himself on fire outside the courthouse where former US president Donald Trump is on trial has died, police said yesterday. The New York City Police Department (NYPD) said the man was declared dead by staff at an area hospital. The man was in Collect Pond Park at about 1:30pm on Friday when he took out pamphlets espousing conspiracy theories, tossed them around, then doused himself in an accelerant and set himself on fire, officials and witnesses said. A large number of police officers were nearby when it happened. Some officers and bystanders rushed

HYPOCRISY? The Chinese Ministry of Foreign Affairs yesterday asked whether Biden was talking about China or the US when he used the word ‘xenophobic’ US President Joe Biden on Wednesday called for a hike in steel tariffs on China, accusing Beijing of cheating as he spoke at a campaign event in Pennsylvania. Biden accused China of xenophobia, too, in a speech to union members in Pittsburgh. “They’re not competing, they’re cheating. They’re cheating and we’ve seen the damage here in America,” Biden said. Chinese steel companies “don’t need to worry about making a profit because the Chinese government is subsidizing them so heavily,” he said. Biden said he had called for the US Trade Representative to triple the tariff rates for Chinese steel and aluminum if Beijing was

Beijing is continuing to commit genocide and crimes against humanity against Uyghurs and other Muslim minorities in its western Xinjiang province, U.S. Secretary of State Antony Blinken said in a report published on Monday, ahead of his planned visit to China this week. The State Department’s annual human rights report, which documents abuses recorded all over the world during the previous calendar year, repeated language from previous years on the treatment of Muslims in Xinjiang, but the publication raises the issue ahead of delicate talks, including on the war in Ukraine and global trade, between the top U.S. diplomat and Chinese