The company that produced contaminated medications linked to an unprecedented fungal meningitis outbreak in the US faced mounting scrutiny on Saturday over whether it illegally sold drugs to medical facilities, as the death toll from the disease grew to 15.
The US Centers for Disease Control and Prevention (CDC) said another person died from meningitis, the second death in Indiana.
The number of cases of the disease reported reached 201 in 14 states, according to the CDC and state officials.
Illinois reported its first case of meningitis from a steroid injection and New Hampshire officials reported that state’s first four confirmed cases from the outbreak, which showed no signs of abating.
Tennessee is the worst affected state with six deaths and 52 cases, followed by Michigan with three deaths and 41 cases, including one case of an infection that has not been confirmed as meningitis.
As federal and state authorities scrambled to contain the outbreak, investigators were trying to determine how the medication produced by New England Compounding Center (NECC) was contaminated and whether its sprawling drug supply business complied with licensing laws.
A series of e-mails between the company and a clinic in Mississippi reviewed by reporters show that NECC sold drugs without requiring physicians to supply individual patient prescriptions. The customer confirmed that NECC supplied the clinic with drugs without patient names or prescriptions, which are required by a number of states including Massachusetts, where the company is based.
The e-mails also indicate that NECC referred business to a sister company, Ameridose LLC, despite a statement by Ameridose last week that the two operated separately. NECC has recalled the suspect product, surrendered its license to operate in Massachusetts and suspended operations. Ameridose also has temporarily suspended operations.
“NECC’s intent has always been to operate in compliance with our licenses in the states where we do business,” the company said in a statement.
The US Food and Drug Administration is investigating NECC and there have been calls from some in Congress for a criminal investigation of the company.
“FDA considers this to be one of our top priorities and we are dedicating many resources to this investigation,” the agency said in a statement late on Friday.
Federal regulators have come under criticism for failing to prevent the outbreak by closely regulating drug compounding companies such as NECC, which prepare medications for clinics and doctors largely outside federal oversight. The FDA has said the law does not give it adequate authority to do so, leaving regulation largely to the states.
NECC faces mounting threats from states as well. Several states are investigating the company and at least two — Michigan and Massachusetts — have said the company violated their regulations, according to a survey.
About 14,000 patients received the suspect steroid medications, which were shipped to 76 facilities in 23 states as long ago as May.
Meningitis is an infection of the membranes covering the brain and spinal cord. Symptoms include headache, fever and nausea. Fungal meningitis is a rare form and is not contagious.
Nearly half of China’s major cities are suffering “moderate to severe” levels of subsidence, putting millions of people at risk of flooding, especially as sea levels rise, according to a study of nationwide satellite data released yesterday. The authors of the paper, published by the journal Science, found that 45 percent of China’s urban land was sinking faster than 3mm per year, with 16 percent at more than 10mm per year, driven not only by declining water tables, but also the sheer weight of the built environment. With China’s urban population already in excess of 900 million people, “even a small portion

‘IN A DIFFERENT PLACE’: The envoy first visited Shanghai, where he attended a Chinese basketball playoff match, and is to meet top officials in Beijing tomorrow US Secretary of State Antony Blinken yesterday arrived in China on his second visit in a year as the US ramps up pressure on its rival over its support for Russia while also seeking to manage tensions with Beijing. The US diplomat tomorrow is to meet China’s top brass in Beijing, where he is also expected to plead for restraint as Taiwan inaugurates president-elect William Lai (賴清德), and to raise US concerns on Chinese trade practices. However, Blinken is also seeking to stabilize ties, with tensions between the world’s two largest economies easing since his previous visit in June last year. At the
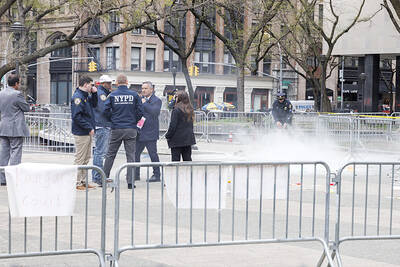
UNSETTLING IMAGES: The scene took place in front of TV crews covering the Trump trial, with a CNN anchor calling it an ‘emotional and unbelievably disturbing moment’ A man who doused himself in an accelerant and set himself on fire outside the courthouse where former US president Donald Trump is on trial has died, police said yesterday. The New York City Police Department (NYPD) said the man was declared dead by staff at an area hospital. The man was in Collect Pond Park at about 1:30pm on Friday when he took out pamphlets espousing conspiracy theories, tossed them around, then doused himself in an accelerant and set himself on fire, officials and witnesses said. A large number of police officers were nearby when it happened. Some officers and bystanders rushed

Beijing is continuing to commit genocide and crimes against humanity against Uyghurs and other Muslim minorities in its western Xinjiang province, U.S. Secretary of State Antony Blinken said in a report published on Monday, ahead of his planned visit to China this week. The State Department’s annual human rights report, which documents abuses recorded all over the world during the previous calendar year, repeated language from previous years on the treatment of Muslims in Xinjiang, but the publication raises the issue ahead of delicate talks, including on the war in Ukraine and global trade, between the top U.S. diplomat and Chinese