More than 250 British women are taking court action after more than half experienced ruptures in breast implants made by a French company at the center of a cancer scare, a lawyer said on Wednesday.
The women are among up to 50,000 in Britain who have had implants that were manufactured by the now-bankrupt Poly Implant Prothese (PIP).
Health officials in France have said the government plans to recommend to 30,000 French women with PIP implants that they have them removed, after eight cases of cancer, mainly breast cancer, were reported.
A lawyer representing more than 250 women in Britain said legal proceedings would start next year, with the complainants making claims against the clinics which carried out the operations to insert the implants.
“Over half of these women have suffered ruptured implants and we are also representing other women who are worried by the reports of problems and worried that their implants could rupture eventually,” lawyer Esyllt Hughes said. “We have issued some court proceedings and we expect them to begin in Cardiff next year.”
Documents obtained on Wednesday showed that tens of thousands of women in more than 65 countries, mainly in South America and western Europe, received implants produced by PIP, which ceased trading last year.
European authorities sought on Wednesday to head off panic over the scare, saying there was no proof of a link to cancer.
However, the French health ministry has said there is no “urgent health risk” from the implants and no “causal link” with cancer has yet been proved.
An expert report will be released in France today saying whether the implants should be removed.
PIP was shut down and its products banned last year after it was revealed to have been using non-authorized silicone gel that caused abnormally high rupture rates of its implants.
Facing financial difficulties, the company, once the world’s third-largest producer of silicone implants, replaced the medical-grade silicone in its implants with industrial-strength material.
The Medicines and Healthcare products Regulatory Agency (MHRA) in Britain urged patients not to panic, although it said they may want to consult their surgeons.
“We did extensive genotoxic and chemical tests, and we could find no evidence of any safety aspect associated with this filler,” MHRA medical director Suzanne Ludgate told BBC radio. “We have been working very closely with the professional bodies to look at the incidence of cancer associated with these breast implants and we’ve worked with the cancer registry, and we can find no evidence for any association.”
Prosecutors in Marseille, near the firm’s home base of Seyne-sur-Mer, have received more than 2,000 complaints from French women who received the implants and they have opened a criminal investigation into the firm.
Yves Haddad, a lawyer for 72-year-old PIP founder Jean-Claude Mas, said his client was prepared to face prosecution and denied the implants could be linked with health problems.
“For the moment, there is no evidence that the product can cause illness,” the lawyer said.
Nearly half of China’s major cities are suffering “moderate to severe” levels of subsidence, putting millions of people at risk of flooding, especially as sea levels rise, according to a study of nationwide satellite data released yesterday. The authors of the paper, published by the journal Science, found that 45 percent of China’s urban land was sinking faster than 3mm per year, with 16 percent at more than 10mm per year, driven not only by declining water tables, but also the sheer weight of the built environment. With China’s urban population already in excess of 900 million people, “even a small portion
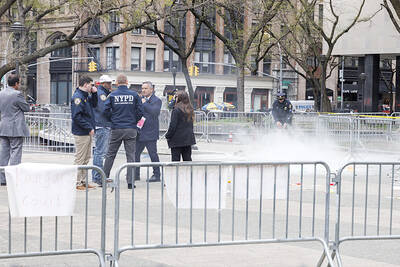
UNSETTLING IMAGES: The scene took place in front of TV crews covering the Trump trial, with a CNN anchor calling it an ‘emotional and unbelievably disturbing moment’ A man who doused himself in an accelerant and set himself on fire outside the courthouse where former US president Donald Trump is on trial has died, police said yesterday. The New York City Police Department (NYPD) said the man was declared dead by staff at an area hospital. The man was in Collect Pond Park at about 1:30pm on Friday when he took out pamphlets espousing conspiracy theories, tossed them around, then doused himself in an accelerant and set himself on fire, officials and witnesses said. A large number of police officers were nearby when it happened. Some officers and bystanders rushed

Beijing is continuing to commit genocide and crimes against humanity against Uyghurs and other Muslim minorities in its western Xinjiang province, U.S. Secretary of State Antony Blinken said in a report published on Monday, ahead of his planned visit to China this week. The State Department’s annual human rights report, which documents abuses recorded all over the world during the previous calendar year, repeated language from previous years on the treatment of Muslims in Xinjiang, but the publication raises the issue ahead of delicate talks, including on the war in Ukraine and global trade, between the top U.S. diplomat and Chinese

HYPOCRISY? The Chinese Ministry of Foreign Affairs yesterday asked whether Biden was talking about China or the US when he used the word ‘xenophobic’ US President Joe Biden on Wednesday called for a hike in steel tariffs on China, accusing Beijing of cheating as he spoke at a campaign event in Pennsylvania. Biden accused China of xenophobia, too, in a speech to union members in Pittsburgh. “They’re not competing, they’re cheating. They’re cheating and we’ve seen the damage here in America,” Biden said. Chinese steel companies “don’t need to worry about making a profit because the Chinese government is subsidizing them so heavily,” he said. Biden said he had called for the US Trade Representative to triple the tariff rates for Chinese steel and aluminum if Beijing was