The Ministry of Health and Welfare has decided not to use two batches of influenza vaccine in the national vaccination program, after doses with the same lot numbers were found to be flawed over the past week.
The decision was made on Thursday during a ministry meeting on flu control, which was to decide about the two discolored doses reported on Wednesday last week and on Monday.
Flaws were found in one dose of Vaxigrip flu vaccine made by French manufacturer Sanofi Pasteur and a dose of children’s flu vaccine manufactured by Taiwan-based vaccine maker Adimmune Corp.
Upon the discovery, the ministry ordered an immediate suspension of all doses with the same lot numbers and launched checks in cooperation with the manufacturers.
“Nothing unusual was detected,” said Lee Ping-ying (李秉穎), a pediatrician at National Taiwan University Hospital and convener of the inoculation consultant team serving the ministry.
The checks found that the flaws were not due to production problems, but resulted from unexpected incidents during the syringe-filling process or the syringe production process, Lee said.
The two problematic doses — one of which was discolored, the other appeared to contain white suspended matter — were determined to be “one-off abnormalities” during the meeting, he added.
Experts nevertheless suggested that the batches be shelved, Lee said.
Adimmune in a statement on Thursday said that the flaw in its product was as an “accident.”
An investigation found no other problematic vaccines in the same lot, the company said, adding that all the doses it produces meet good manufacturing practice standards.
The white suspended matter spotted in its product was confirmed to be a cloud of plastic fragments from the syringe itself, it said.
Sanofi Pasteur said that no further anomalies in its vaccine were found during an inventory check conducted with local health officials.
Centers for Disease Control (CDC) data showed that by Wednesday, 2.38 million doses of flu vaccine had been administered as part of the national inoculation program this year.
Nationwide, 31 cases of people having reactions after receiving the inoculation had been reported, only one of which involved vaccine from the problematic batch FKAE1802, CDC Deputy Director-General Chuang Jen-hsiang (莊人祥) said.
That case was a 22-month-old infant who developed symptoms of diarrhea, throat inflammation and fever one day after getting a flu shot, Chuang said, adding that the patient was later diagnosed with bronchitis and an upset stomach.
The infant has recovered after treatment, he added.
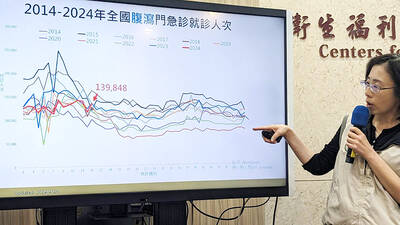
FLU SEASON: Twenty-six severe cases were reported from Tuesday last week to Monday, including a seven-year-old girl diagnosed with influenza-associated encephalopathy Nearly 140,000 people sought medical assistance for diarrhea last week, the Centers for Disease Control (CDC) said on Tuesday. From April 7 to Saturday last week, 139,848 people sought medical help for diarrhea-related illness, a 15.7 percent increase from last week’s 120,868 reports, CDC Epidemic Intelligence Center Deputy Director Lee Chia-lin (李佳琳) said. The number of people who reported diarrhea-related illness last week was the fourth highest in the same time period over the past decade, Lee said. Over the past four weeks, 203 mass illness cases had been reported, nearly four times higher than the 54 cases documented in the same period

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not
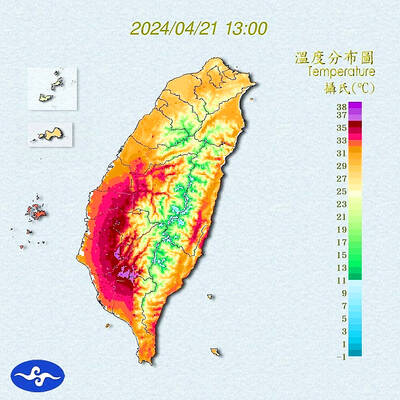
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching