The Taiwan Pharmacist Association yesterday urged authorities to curb the prevalent use of human drugs by veterinarians to treat animals, saying that the practice is unsafe for pets and worsening the problem of antimicrobial resistance.
The Council of Agriculture said earlier last week that it is working with the Food and Drug Administration (FDA) on a list of 654 human drugs to be approved for use by veterinarians, following the 116 human drugs authorized for animal use last year.
The government’s actions followed an incident in which a domestic drugmaker was admonished by health authorities in accordance with the Pharmaceutical Affairs Act (藥事法) over reportedly selling drugs for humans to pet hospitals.
Veterinarians and pet owners were concerned about a possible domino effect that could affect the supply of human drugs, which — according to veterinarians — account for about 80 percent of veterinarian drugs and have been used for animals for more than 50 years in the nation.
The pharmacist association yesterday held a press conference in which members objected to the liberalization of the use of human drugs, saying that the council and the veterinarians “failed to negotiate with domestic veterinarian drug manufacturers for a fitting solution before amending the law ad hoc out of convenience and for cost reduction.”
The pharmacists stated that animal metabolism and physiology differ from those of humans, and drugs for humans are made with calculated doses and supervised processes according to human needs.
“Drug resistance is another potential problem,” said Chiu Chien-chiang (邱建強), executive director of the Taichung County Pharmacist Association.
“According to an article in the Infection Control Journal, [published by the Ministry of Health and Welfare and the Infection Control Society of Taiwan,] as much as 70 to 76 percent of the antibiotics used in the country have been used in animals, a figure that is much higher than the 48 percent in the EU,” Chiu said.
Studies have suggested that antibiotic resistance might have been caused by the use of the drugs in animals as growth promoters or for treatment, Chiu said.
“In the EU, about 25,000 people die from multidrug-resistant infections every year, with the related expenses amounting to 1.5 billion euros,” he added.
Chiu Chui-chang (邱垂章), an official at the council’s Bureau of Animal and Plant Health Inspection and Quarantine, said that the referenced antibiotics are mainly used in livestock, through their feed.
“We have started to take measures to rein in the practice,” Chiu Chui-chang said.
“Pets, on the other hand, do not receive antibiotics except during a course of treatment. They are more likely to receive other kinds of human drugs, such as those for treating cardiovascular or kidney diseases,” the official added.
“The use of human drugs for pet animals is commonly seen in other developed countries such as the EU, the US and Japan. The source of pharmaceuticals is the same, while two licenses are issued,” Chiu Chui-chang said.
Taipei Veterinary Medical Association president Simon Yang (楊靜宇) agreed, adding that in the US, veterinarian and human drugs are both under supervision of the [US] FDA, while Taiwan has the council covering animal drugs and the FDA responsible for human drugs, hampering possible internal discussion.
Chiu Chien-chiang said pharmacists worry about the current lack of supervision and controlled management of the drug use.
Chiu Chui-chang said that the council will work with the FDA to build a professional evaluation and approval system overlooking the use of human drugs in animals.
“The approved list will exclude those having sufficiently obtainable veterinarian counterparts,” Chiu said.

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not
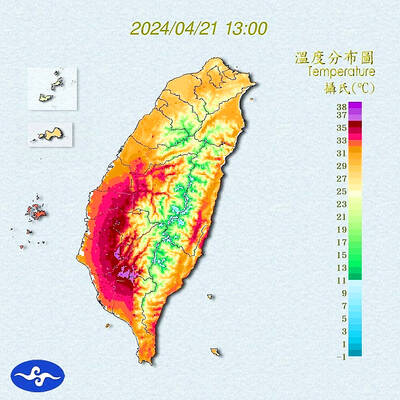
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater