Products containing rhododendrol have been banned following complaints from consumers about depigmentation of the skin after they had used a product produced by Kanebo Cosmetics, the Food and Drug Administration (FDA) said on Tuesday last week.
The administration said that it had received many complaints from consumers that a Kanebo product containing rhododendrol had caused temporary depigmentation in the form of spots that appeared on their skin.
Kanebo is the only cosmetic company known to have used the substance in its products and following complaints the company’s Taiwan branch started a general recall of its products in Asia on July 4. By the end of last month, a total of 63,784 bottles, or about 99 percent of the product, had been recalled, the agency said.
Kanebo said the product contains natural minerals that help in the repression of melanin production, adding that the company spent 11 years researching the product.
The company also said that the product had passed a Japanese Ministry of Health, Labor and Welfare inspection.
Dermatologists said the depigmentation may have been due to an unforeseen allergic reaction to rhododendrol or simply because the substance is too effective.
The repression of melanin production by rhododendrol is 25 times that of vitamin C and as the substance is “toxic” to melanin, the depigmentation may simply be excessive repression, dermatologists said.
Food and Drug Administration medical supplies and cosmetic equipment division chief Lu Li-fu (呂理福) said the agency had received 354 complaints, with about 276 of those suspected of exhibiting depigmentation of the skin due to the use of the Kanebo product.
The other 47 were diagnosed as having depigmentation caused by other chemical substances, Lu said.
The depigmentation should disappear as long as patients diagnosed with the condition cease to use the product, Lu said, adding that some may require a brief period of medication before the effects of the depigmentation wear off.
The agency said that after extensive research into rhododendrol, it has banned its use in cosmetic products.
The agency said products containing the substance can no longer be imported or manufactured.
Additional reporting by Hung Su-ching

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not
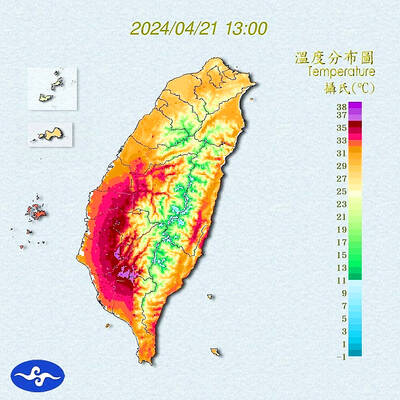
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater