Private umbilical cord blood banks making exaggerated medical promises face stiff fines, the Food and Drug Administration (FDA) said yesterday in response to a recent case of the mother of a paralyzed child accusing a private cord blood bank of leading her to believe that spinal surgery using the cord blood was a viable cure for her daughter.
The mother had stored the cord blood of her three-year-old daughter Hsun-hsun, who has tetraplegia, at the cost of NT$75,000 three years ago.
However, when the mother asked a hospital to perform an operation using stem cells derived from umbilical cord blood, the hospital said that the operation would be the nation’s first so the permission of the Ministry of Health and Welfare was needed.
However, the surgery did not gain approval from the health authorities, who said that it would be illegal and the hospital was not technologically advanced enough to perform such a procedure.
Hsun-hsun’s mother was outraged and accused the salesperson of the cord blood bank of overstating the effectiveness of cord blood and deceiving consumers.
“I paid the bank to store the cord blood hoping that my child would be able to use it someday if she needed it. If it is not usable, why does the government allow [private blood banks] to sell such services?” she said.
The FDA said that under current regulations, cord blood banking facilities must not make any promises about procedures involving cord blood expected to be developed in the future.
As for the reports by local media saying that some cord blood salespeople had made false claims about 3D printing of human organs in five to 10 years or having the ability to make eight bags of cord blood out of one, the FDA said that the company would be subject to a fine of up to NT$500,000 in accordance with the Regulations for Administration on Human Organ Bank (人體器官保存庫管理辦法) and the Organ Transplant Act (人體器官移植條例).
The Consumer Protection Act (消費者保護法) and the Fair Trade Act (公平交易法) might also apply if the violations are reported to the relevant authorities by the consumers, the administration said.
However, the government might be able to do more than just fining the companies or reining in their claims.
Mark Liu (劉宏恩), a law professor at National Chengchi University specializing in biomedical ethics, has a different take on the incident.
Describing the commercialization of cord blood banking in Taiwan as “going beyond the pale,” Liu said the lesson that should be drawn from the incident is the point mentioned by the mother on the uselessness of the privately banked cord blood.
Liu said the government was ignoring the fact that many European countries have either restricted or banned the commercialization of cord blood banking and that Japan has only public cord blood banks.
“Their logic is simple,” Liu said. “They believe that private cord blood banking is against the public’s interest.”
“Cord blood can be matched to different people, which is to say that, just as my cord blood might help save somebody else’s life, my life can be saved by somebody else’s cord blood. So the idea of just storing my own blood for my own use doesn’t make sense at all,” Liu said, adding that according to the US’ National Institutes of Health, the odds of successfully using a person’s own cord blood for medical procedures in his or her lifetime may be as low as one in 200,000.

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not
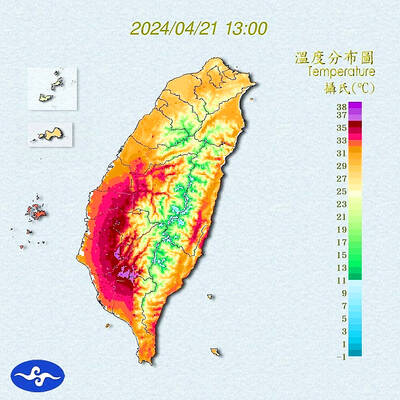
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater