With temperatures dropping in recent weeks, consumers should pay close attention to the labeling on “hot spring” powders, as only one out of 44 products recently tested by the Consumer Protection Committee was found to actually contain substances extracted from thermal spring water, while the rest contained a mixture of chemicals and bath salts.
In October, the committee conducted tests on 44 hot spring powders, some claiming to have curative effects, purchased from shopping centers and department stores in seven cities and counties.
Apart from a bath powder imported from Japan by Taipei-based Yuantai Trading Co, the others were mainly comprised of chemicals such as sodium hydrogen carbonate and sodium sulfate, as well as bath salts, according to test results released on Monday.

Photo: Liu Hsin-de, Taipei Times
“Customers who purchase such products may end up bathing in water laced with chemicals,” consumer protection official Wang Teh-ming (王德明) said.
Some of the products tested exaggerated their effects or provided deceitful labeling information, which not only misleads customers, but also constitutes violations of several regulations, the report said.
A product imported by Adoration International Co, which claimed curative effects for sprains, eczema, neuropathic pain and hemorrhoids, was found to be in violation of Article 69 of the Pharmaceutical Affairs Act (藥事法), Food and Drug Administration Director Lin Cheng-chin (林澄琴) said.
The article forbids advertisements or labels regarding the medical efficacy of any products that are not defined in the act as medications
In response, Adoration proprietor Lin Ching-kuo (林進國) said the product’s claimed medical capabilities were direct translations from the packaging of its Japanese manufacturer and pledged to recall the product and make the necessary corrections.
Three other items tested which trumpeted the effects of stimulating blood circulation and metabolism may also contravene the Fair Trade Act (公平交易法) by bearing false and exaggerated advertising labels, Lin Cheng-chin said. Transgressions are punishable by a maximum fine of NT$25 million (US$783,000).
Additionally, five products manufactured by Tengchi Ltd could incur fines of up to NT$100,000 as stipulated by the Statute for Control of Cosmetic Hygiene for listing vitamin C, milk extracts and natural mineral substances among their ingredients, none of which were found in tests, the report said.
When reached for comment, an accountant at Tengchi, surnamed Wang (王), said: “How could [the tested products] possibly contain milk extracts, as the term only refers to the fragrance of the product itself? We will pull the products in question off the shelves for re-packaging.”
Meanwhile, one product was found to have allegedly misappropriated the name of Changhua-based Shoufu Biotech Co as its manufacturer. Shoufu owner Liu Yi-cheng (劉怡成) has signed an affidavit saying that the company’s shipment records prove that the company was not the producer of the products.
Urging people to wash with clean water after using such products to prevent harmful substances left on the skin from further oxidizing, Gaston Wu (吳家誠), a professor in chemistry at National Taiwan Normal University, said soaking in water containing sodium hydrogen carbonate and sodium sulfate could lead to skin inflammation and infection.
“Since children have less resistance to disease, parents should be more careful,” Wu said.
Wu said that since most hot spring water in the country is at high temperature and under strict control, it is extremely difficult to acquire thermal water and have it processed into powders.
“Domestic manufacturers who claim their hot spring bath powders are extracted from hot spring water may actually use ‘second-hand thermal spring water’ in which people have bathed,” Wu said.
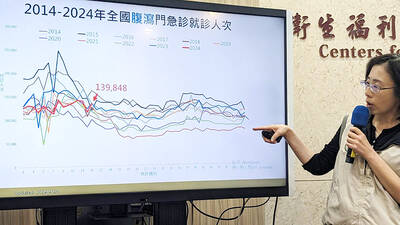
FLU SEASON: Twenty-six severe cases were reported from Tuesday last week to Monday, including a seven-year-old girl diagnosed with influenza-associated encephalopathy Nearly 140,000 people sought medical assistance for diarrhea last week, the Centers for Disease Control (CDC) said on Tuesday. From April 7 to Saturday last week, 139,848 people sought medical help for diarrhea-related illness, a 15.7 percent increase from last week’s 120,868 reports, CDC Epidemic Intelligence Center Deputy Director Lee Chia-lin (李佳琳) said. The number of people who reported diarrhea-related illness last week was the fourth highest in the same time period over the past decade, Lee said. Over the past four weeks, 203 mass illness cases had been reported, nearly four times higher than the 54 cases documented in the same period

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:
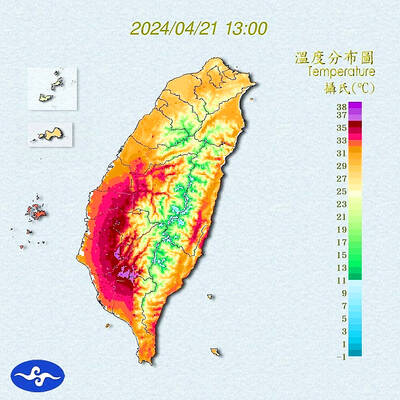
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not