The American Institute in Taiwan (AIT) yesterday provided information it said proved that US beef is safe for consumption in response to what it called “misinformation” regarding the products that have been widely circulated recently.
Spokesperson Sheila Paskman said in a press statement that the AIT has been “very concerned about the extensive misinformation.”
Therefore, the AIT provided the documents “to give the straightforward facts about US beef,” Paskman said.
“We hope you will read this and understand the basis for our stance on the safety of US beef,” she said.
Recent press reports about health problems in animals that are fed ractopamine misrepresented the data from these studies and failed to make any legitimate link to health risks in humans, the AIT said.
Since ractopamine was approved for pigs in 1999 and for cattle in 2003, there have been billions of kilos of meat from animals fed with ractopamine consumed by hundreds of millions of people during that period, it said.
“There have been no reports of any human illness linked to the consumption of beef or pork from animals that have been fed ractopamine,” it said.
Major beef-producing or importing countries, including Japan, South Korea, Mexico, Canada and many others, have also determined that meat from animals that are fed ractopamine is safe for human consumption, the statement said.
There have been extensive scientific studies that reviewed the use of ractopamine as a feed ingredient and considered its impact on human health in terms of toxicity, reproductive abnormalities, carcinogenicity and other factors, including the Joint Expert Committee on Food Additives (JECFA), which operates under the Food and Agriculture Organization and the WHO, along with 27 countries that had conducted government-directed risk assessments, the AIT said.
Taiwan has also conducted its own risk assessment and in 2007 notified the WTO of its intention to establish maximum residue levels (MRL) for ractopamine in beef and pork.
“Taiwan’s own testing of imported meat products confirmed that ractopamine residues in US beef fell well within the MRLs recommended by JECFA, which are the same draft MRLs that Taiwan notified to the WTO in 2007,” it said.
In response to critiques of the JECFA assessment that said that a single human study with only six test subjects was used to support the committee’s MRL recommendations for ractopamine, the AIT said that “in fact, JECFA conducted multiple reviews of the use of ractopamine in animals and based its recommendations on more than 20 animal studies, including long-term studies with primates, in addition to the human study.”
“It would be highly unusual to take the extra step of testing humans with drugs that are intended for use in animals, and therefore the sample size for the human study was limited for ethical reasons. The human study was intended only to verify that humans react to ractopamine in a way similar to primates, so that the levels determined to be safe for primates could help determine safe levels for humans,” the AIT said.
JECFA’s findings were sufficient to demonstrate the safety of ractopamine, but due to opposition from some members of the Codex Alimentarius Commission (CAC), the international regulatory body for food safety, primarily the EU and China, the CAC could not reach a consensus, the AIT said.

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not
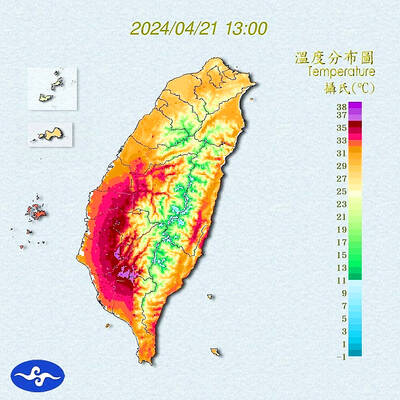
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater