Liberty Times: When Yu Chang Biologics Co, now known as TaiMed Biologics Co, was created to import the TNX-355 drug, the participants wished to make it an example for the biotech industry. However, some are questioning whether the process of acquiring assistance from the National Development Fund [NDF] went so smoothly because there might be have been an “abuse of privilege.” What are your thoughts on this?
Patrick Yang [楊育民]: In January 2007, Academia Sinica President Wong Chi-huey [翁啟惠] and [then] National Science Council minister Chen Chien-jen [陳建仁] hosted a Northern Hsinchu BioMed Park Area Steering Committee meeting in which the participants discussed a general blueprint for the biotech industry. They touched on matters regarding research and development [R&D], clinical tests, legal statutes, funding issues and the establishment of protein factories, in hopes of finding a possible plan with the potential to go from R&D through to manufacturing.
At the time, I was executive vice president of product operations at [US biotech firm] Genentech Inc and the deal to acquire Tanox and its [second-phase clinical trial] TNX-355 had been certified. I told Wong of the news, and the Executive Yuan decided to make a bid for it in February.
At the time, there were already about 10 countries and corporations that had professed an interest in the new drug, with three corporations even offering to buy the drug, so Taiwan was under pressure from a lot of international competition.
At the time, Taiwan did not have a complete set of legal regulations concerning human testing, biotech investment and fundraising.
Wong and then-minister without portfolio Ho Mei-yueh [何美玥], and even then-deputy premier Tsai Ing-wen [蔡英文], spared no efforts in cementing the foundations that went on to become TaiMed, which then made a bid for and succeeded in acquiring the patent rights to TNX-355 in August 2007.
This all went through discussion and planning that lasted for months, while competing with international companies and negotiating with Genentech, as well as taking measures to accommodate Taiwan’s administrative procedural limitations. The NDF was only one checkpoint on the procedural path, and it was also assessed and reviewed by the government; in any case, we were racing against time.
In other countries, making a bid for patent rights is seizing an opportunity, but in Taiwan, it’s being described as an “abuse of privilege.” It’s incomprehensible.
LT: The founders of Tanox mentioned having applied to the NDF about chipping in and mass producing TNX-355, but their application was rejected, while TaiMed’s TNX-355 application passed. How would you view the two cases?
Yang: I haven’t seen the complete contents of the Nanhwa case [the failed bid for NDF funding by Taiwan Biopharmaceuticals Co, a company invested in by Tanox and then-Euroc Venture Capital Co chairman Kao Yu-jen [高育仁] for the research and development of TNX-355], but from the information gleaned from the various other sectors, the two cases are in actuality incomparable.
Taiwan Biopharmaceuticals Co was applying for an NT$1.7 billion [US$56 million] investment by the NDF and spending NT$7 billion to NT$8 billion on building a factory. But, at that time, it wasn’t even certain what sort of drugs would be mass produced.
Yu Chang, on the other hand, applied for investment from the NDF amounting to US$20 million, approximately NT$600 million — of which the NDF only invested NT$400 million — while Yu Chang raised US$50 million from the private sector to buy the patent rights to TNX-355 with the intent of starting from human test research while developing “drug manufacturing technology.”
Besides, Yu Chang was waiting for the success of third-phase clinical trials of the drug before contemplating building any factories; the purpose and use of investment were completely different.
When Taiwan Biopharmaceuticals Co applied for the case in 2005, TNX-335 was still in the first-phase of clinical testing and the investment risk was too great to build a factory. When Yu Chang applied for investment in 2007, the drug was already in second-phase clinical testing, with a higher chance of success, smaller amount of investment funds needed and lower risks.
More importantly, Yu Chang had two major things to their advantage — the first being the involvement of David Ho [何大一], a world-class AIDS expert, which would significantly raise the success rate of the drug, and the second being that Yu Chang’s cooperation partner was Genentech, the prime global biologic drug company, with more than 11,000 workers, making US$11.7 billion per year and with six drug factories worldwide.
Tanox only had 500 workers and made US$45 million per year at the time.
LT: Ho participated in the review of the Nanhwa case and had once objected to the TNX-355 drug, but he later helped Yu Chang produce TNX-355. Why [do you think] he later supported [the production of the drug]?
Yang: When Wong made the evaluation of whether to make a bid for TNX-355 as part of the biotech pioneering plan, he consulted Ho on drug. Ho was initially against it because he felt that even if TNX-355 was successfully manufactured, it would be in the back-line “therapeutic medication;” there would not be a large enough market to stimulate Taiwan’s biotech drug production industry.
There were also other international companies that were doing similar research. I think that was one of the reasons why he opposed the Nanhwa case, because he was afraid TNX-355 would not be able to [generate enough sales] to pay back the investment of building a factory.
Luckily, Ho was finally persuaded, and with his experience and research in the AIDS field, the research could further expand TNX-355 into a “preventive” medication, creating a market and sales viability. This gave him confidence that Taiwan’s investment would be paid back.
LT: Some have said that TaiMed caused the NDF to lose money, but Ho holds joint venture shares. Is this a special exception in the field, or a common international occurrence?
Yang: Some people keep saying that TaiMed is losing money, saying profits of billions of New Taiwan dollars based on prices of emerging stocks can’t be counted toward actual prices, but these people are also using the same emerging stock prices to calculate Ho’s shares and saying Ho made billions of dollars. The logic is contradictory, and it is also the opinion of laymen.
The bio-pharmaceutical field has different prices at each level of R&D. Taiwan has never actually conducted a human experiment and there are no regulations stating the value of each level of human experimentation, but internationally it’s a commodity that could be given a price.
TaiMed’s TNX-355 is about to start third-phase human experimentation, and the price is already adding up.
Even the KMT [Chinese Nationalist Party] administration was willing to increase its investment in the project by another NT$130 million.
What some people are calling Ho’s joint venture stocks were stocks given to Ho by the Ruentex Group [潤泰集團], who bought the shares originally belonging to Tsai’s family [one of the founding partners of the company].
All of the board members, including the representatives of the NDF, agreed to the motion.
Ho’s participation in the R&D process of TNX-355 not only bumped the drug from a therapeutic drug to a preventive drug, but also — because he made the call for hospitals, foreign and domestic, to cooperate on human experimentation — gave Taiwan [the chance] to participate formally in drug manufacturing and experimentation. It is also because of Ho that the Bill Gates Foundation agreed to invest in TaiMed’s TNX-355 project, making TaiMed the first company in Taiwan to receive an investment from Gates.
Recently, the health of Ho’s mother has not been too good and he is focusing all his attention on taking care of her. When speaking of these strange accusations, he was always of the mind that it was not worth responding to, only slightly lamenting that he was being vilified for trying to do something good for his native country.
LT: After Tsai quit as deputy premier, she assumed the position of TaiMed chairwoman. Though [she] made certain she did not violate the “revolving door” clause, the incident became the focus of public attention three years later. Do you still believe that she was an appropriate candidate for chairperson of TaiMed?
Yang: When TaiMed was first created, Tsai had not considered any position within the company. It was only after she resigned as deputy premier. And because Ho was unable to become chairperson and because of the difficulty in raising funds from the private sector, Wong approached Tsai for help.
Account information from TaiMed also showed that if it were not for her help in raising the funds and wiring the first deposit into the account on Sept. 3, 2007, then the subsequent wiring of funds from the NDF and the President International Development Corporation and others, TaiMed would not have been able to buy the patent rights to TNX-355 in time.
It is also because of Taiwan’s bid for TNX-355 that I came into contact with Tsai and made in-depth observations about her.
I saw that she worked hard to understand the biotech industry and was calm and decisive in her judgement and negotiation skills when she made international negotiations, administrative coordination, as well as raising necessary funds.
Add to that her poise under the current political pressure, I am convinced Tsai would make an extraordinary leader. She has morals and is trustworthy and capable. Her decisionmaking, negotiation and coordination skills impressed me. She can shoulder responsibility and also has guts, and places the benefits of the team and the nation first, and is a leader that could be trusted.
LT: Both the Democratic Progressive Party and the KMT said they made the biotech sector a main focus of policymaking. With the incessant political infighting facing Taiwan’s biotech industry, does it still have a chance?
Yang: The most important thing for a nation if it wants to nurture a particular industry is the attitude of the government. The current CEPD [Council for Economic Planning and Development] chairperson [Minister Christina Liu (劉憶如)] said that she can’t understand the content of the many documents concerning TaiMed; I would suggest she finds real experts to have a look at it. For instance, the convener of Taiwan Biologic Foundation TMF, Jang Yue-teh [張有德], the first to answer the call of the NT$60 billion “Elevation Biotech — the Diamond Project” promoted by Ma, is a very exceptional scholar.
As a matter of fact, Jang was also one of the strategy committee members of TaiMed, but was later asked to help the KMT government promote the TMF. The model the TMF is going through and the funding difficulties it met with are almost identical to the troubles that TaiMed went through.
When TaiMed met with troubles, it was a godsend that Tsai came on board to help look for talent and invested her own money, helping TaiMed overcome a difficult time.
In comparison, the TMF is not only having difficulty raising funds from the private sector, the state-owned corporations are also not helping. But [instead of helping] the officials in charge are only trying to find official documents to raise small suspicions against former government officials and scientists.
I’m afraid that the “diamond” of the biotech industry will probably not shine very brilliantly.
If proper concepts of long-term investment and an environment that is not affected by blue or green politics cannot be established, I’m afraid that the Taiwanese biotech industry will only be a dream.
Translated by Jake Chung

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not
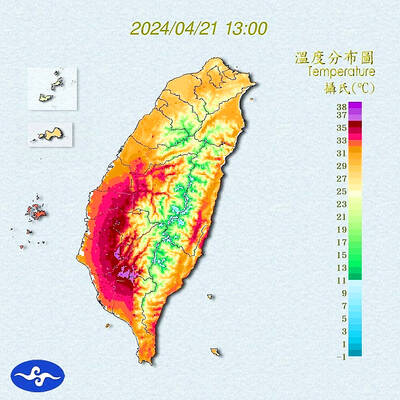
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching

POLICE INVESTIGATING: A man said he quit his job as a nurse at Taipei Tzu Chi Hospital as he had been ‘disgusted’ by the behavior of his colleagues A man yesterday morning wrote online that he had witnessed nurses taking photographs and touching anesthetized patients inappropriately in Taipei Tzu Chi Hospital’s operating theaters. The man surnamed Huang (黃) wrote on the Professional Technology Temple bulletin board that during his six-month stint as a nurse at the hospital, he had seen nurses taking pictures of patients, including of their private parts, after they were anesthetized. Some nurses had also touched patients inappropriately and children were among those photographed, he said. Huang said this “disgusted” him “so much” that “he felt the need to reveal these unethical acts in the operating theater