The Department of Health yesterday issued a warning against Raptiva, a medication used to treat psoriasis, after the drug was linked to a series of reported cases of brain infections.
Since October 2003, the US Food and Drug Administration and European Medicines Agency have been informed of three confirmed cases of progressive multifocal leukoencephalopathy (PML), as well as another possible case, in patients taking Raptiva, foreign news agencies reported yesterday. All four patients had taken Raptiva continuously for at least three years. Three of the patients have died.
Because of safety concerns following the instances of PML, health officials in Europe urged the removal of Raptiva from the market because the benefits of the drug “no longer outweigh its risks,” the agencies reported.
PML is a rare and progressive disease caused by a virus that may lead to an inflammation of the nerves in the brain. Severe cases of PML can lead to paralysis and even death, especially for patients with an immune deficiency.
Taiwan’s Department of Pharmaceutical Affairs yesterday issued a warning that Raptiva may induce the occurrence of PML.
Patients undergoing treatment with Raptiva should be taken off the drug immediately if they display symptoms of disturbances in the nervous system, the department said.
The department has also required the distributor to display a warning on the prescription information for the drug.
Merck Taiwan said that although the company obtained permission to market the drug from the Department of Health two years ago, it has not yet put the drug on the market.
However, “after reports linking [Raptiva] to PML, the company is unlikely to put the drug on market in Taiwan,” a spokesperson said.
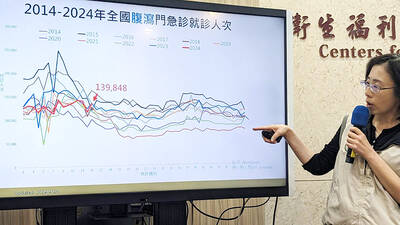
FLU SEASON: Twenty-six severe cases were reported from Tuesday last week to Monday, including a seven-year-old girl diagnosed with influenza-associated encephalopathy Nearly 140,000 people sought medical assistance for diarrhea last week, the Centers for Disease Control (CDC) said on Tuesday. From April 7 to Saturday last week, 139,848 people sought medical help for diarrhea-related illness, a 15.7 percent increase from last week’s 120,868 reports, CDC Epidemic Intelligence Center Deputy Director Lee Chia-lin (李佳琳) said. The number of people who reported diarrhea-related illness last week was the fourth highest in the same time period over the past decade, Lee said. Over the past four weeks, 203 mass illness cases had been reported, nearly four times higher than the 54 cases documented in the same period

A group of Taiwanese-American and Tibetan-American students at Harvard University on Saturday disrupted Chinese Ambassador to the US Xie Feng’s (謝鋒) speech at the school, accusing him of being responsible for numerous human rights violations. Four students — two Taiwanese Americans and two from Tibet — held up banners inside a conference hall where Xie was delivering a speech at the opening ceremony of the Harvard Kennedy School China Conference 2024. In a video clip provided by the Coalition of Students Resisting the CCP (Chinese Communist Party), Taiwanese-American Cosette Wu (吳亭樺) and Tibetan-American Tsering Yangchen are seen holding banners that together read:
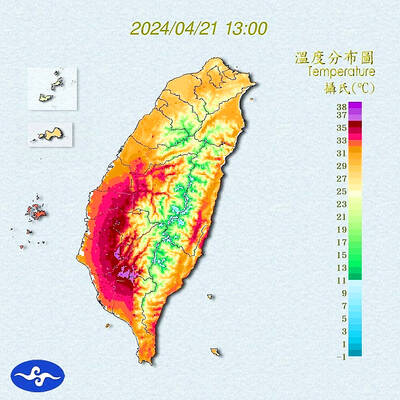
Heat advisories were in effect for nine administrative regions yesterday afternoon as warm southwesterly winds pushed temperatures above 38°C in parts of southern Taiwan, the Central Weather Administration (CWA) said. As of 3:30pm yesterday, Tainan’s Yujing District (玉井) had recorded the day’s highest temperature of 39.7°C, though the measurement will not be included in Taiwan’s official heat records since Yujing is an automatic rather than manually operated weather station, the CWA said. Highs recorded in other areas were 38.7°C in Kaohsiung’s Neimen District (內門), 38.2°C in Chiayi City and 38.1°C in Pingtung’s Sandimen Township (三地門), CWA data showed. The spell of scorching

UNAWARE: Many people sit for long hours every day and eat unhealthy foods, putting them at greater risk of developing one of the ‘three highs,’ an expert said More than 30 percent of adults aged 40 or older who underwent a government-funded health exam were unaware they had at least one of the “three highs” — high blood pressure, high blood lipids or high blood sugar, the Health Promotion Administration (HPA) said yesterday. Among adults aged 40 or older who said they did not have any of the “three highs” before taking the health exam, more than 30 percent were found to have at least one of them, Adult Preventive Health Examination Service data from 2022 showed. People with long-term medical conditions such as hypertension or diabetes usually do not