PharmaEssentia Corp (藥華醫藥) yesterday said that Besremi (Ropeginterferon alfa-2b), its treatment for people with polycythemia vera without symptomatic splenomegaly, has gained marketing approval from the European Medicines Agency.
The company obtained the approval with help from its partner, Austria-based AOP Orphan Pharmaceuticals AG, it said, adding that sales of Besremi in Europe could begin in the first half of this year.
PharmaEssentia has out-licensed to AOP the exclusive rights to market the drug in Europe, the Middle East, the Commonwealth of Independent States and the Baltic Assembly.
PharmaEssentia chief executive officer Lin Ko-chung (林國鐘) in October last year said that the company was preparing to deliver the first batch of 20,000 doses of Besremi to Europe, which would be enough to supply the single market’s estimated 760 patients for a year.
Each dose was expected to retail at US$2,000, Lin said.
However, the company is still finalizing royalty arrangements with AOP before sales can begin in Europe, a company official said yesterday.
Separately, Lotus Pharmaceuticals Co (美時化學製藥) yesterday announced that it has launched a generic equivalent of Suboxone, a sublingual film used to combat opioid addiction in the US.
The treatment, which contains the active ingredients buprenorphine and naloxone administered four times daily under the tongue or inside the cheek, have been made available in four strengths.
The launch took place after the US Supreme Court ruled against UK-based Indivior PLC, Suboxone’s original developer, and opened the way for generics to be sold in the US.
Lotus affiliate Alvogen Group Inc on Jan. 24 obtained final approval from the US Food and Drug Administration for the generic drug and reached an agreement with Indivior on Jan. 31 to lift the restrictions on marketing generics in the US.
Alvogen and Lotus are facing competition from other Suboxone generic makers, Dr Reddy’s Laboratories Ltd of India and Mylan NV registered in the Netherlands.
An estimated 116 Americans die every day from overdoses of prescription painkillers, heroin or synthetic products, such as fentanyl, creating a treatment market estimated at about US$24 billion, the company said last year, citing findings by the US National Center for Health Statistics.
US President Donald Trump has described the issue as an “opioid epidemic” and “health emergency” that requires immediate attention.
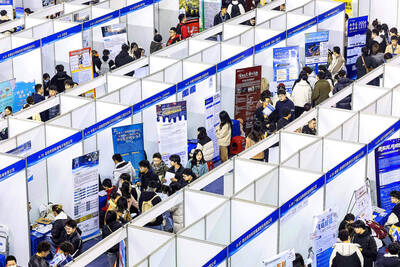
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group
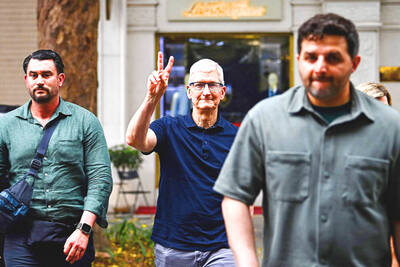
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last