The US Food and Drug Administration (FDA) is on the brink of approving a breakthrough drug that could upend the way severe depression is treated.
Johnson & Johnson’s (J&J) esketamine, a close chemical cousin of the anesthetic ketamine, on Tuesday cleared a major hurdle when a panel of outside experts recommended that the FDA approve the treatment.
The fast-acting antidepressant, administered via a nasal spray, is being tested in major depressive disorder and suicidal thinking.
If approved, it would be the first major therapeutic advance for depression since the introduction of Prozac in 1987.
Prozac and other currently available antidepressants take weeks to work and do not help all patients, so esketamine could mark a significant shift in depression therapy.
The panel voted 14-2 with one abstention that the benefits of the drug and a safety program proposed by the company to keep it from being misused outweigh the risks of abuse.
“I believe esketamine has the potential to be a gamechanger in the treatment of depression,” Walter Dunn, a panel member and a psychiatrist at the West Los Angeles Veterans Administration Medical Center, said after the vote. “The rates of response in this treatment-resistant population is better than we’ve seen. The rapid time line of response is better. There’s nothing approved that gets patients better this fast.”
Amid the opioid addiction and overdose epidemic in the US, the panel of experts weighed the abuse potential of ketamine, which at much higher doses is a party drug and can put users into a “k hole,” in which they are unable to interact with the world around them.
In a report ahead of the meeting, agency staff called ketamine abuse “relatively uncommon,” with just 1.3 percent of people over age 12 abusing the drug, lower than the abuse rates for other hallucinogens like ecstasy and LSD.
“Ketamine is a nasty drug,” said Steve Meisel, a panel member and system director of medication safety at Fairview Health Services in Minneapolis. “It’s been around for 50 years. Those of us who have seen it used know the adverse-event profile is large.”
However, Meisel said he was convinced by a patient survey Johnson & Johnson conducted.
“We don’t take the patient voice into account enough,” he said.
Some patients taking esketamine experienced disassociation, an out-of-body experience that the company said cropped up within an hour of treatment and would be monitored in an office setting if it occurs.
Some patients also experienced modest spikes in blood pressure during that window.
Esketamine was developed by the company after a group of researchers discovered that ketamine, an off-patent drug, had a surprisingly rapid antidepressant effect.
Some of the first research showing this dates back to the 1990s, and that work was furthered by the National Institutes of Health before being developed into a pharmaceutical treatment by J&J.
Studies have also shown ketamine has the potential to rapidly reduce suicidal thinking and J&J is studying esketamine in depressed patients on the verge of killing themselves as well.
That data are expected later this year.
The nasal spray is a key part of J&J’s pharmaceutical pipeline, as the company faces flagging sales weighed down by drug-pricing scrutiny and biosimilar competition for one of its biggest drugs, the blockbuster arthritis drug Remicade.
A decision on whether to allow the drug on the market is expected by March 4.
Esketamine has the FDA’s breakthrough-therapy designation in treatment-resistant depression as well as for depressed people at risk of suicide.
Rival Allergan PLC is also testing a fast-acting antidepressant, rapastinel, which is about a year behind esketamine in testing.
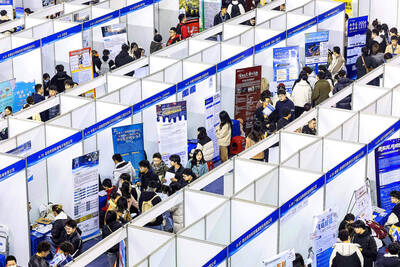
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
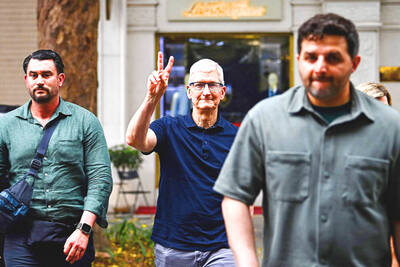
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last

US CONSCULTANT: The US Department of Commerce’s Ursula Burns is a rarely seen US government consultant to be put forward to sit on the board, nominated as an independent director Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday nominated 10 candidates for its new board of directors, including Ursula Burns from the US Department of Commerce. It is rare that TSMC has nominated a US government consultant to sit on its board. Burns was nominated as one of seven independent directors. She is vice chair of the department’s Advisory Council on Supply Chain Competitiveness. Burns is to stand for election at TSMC’s annual shareholders’ meeting on June 4 along with the rest of the candidates. TSMC chairman Mark Liu (劉德音) was not on the list after in December last