PharmaEssentia Corp (藥華醫藥) yesterday held a commencement ceremony for a new fill-and-finish facility as it forges ahead with mass production and commercialization of ropeginterferon alfa-2b (Ropeg), its biologic drug to treat polycythemia vera, a rare type of blood cancer.
The facility in the company’s EU Good Manufacturing Practice-certified manufacturing plant in Taichung’s Central Taiwan Science Park is capable of making 26,000 prefilled vials of Ropeg daily.
The vial production line is to use automated filling line machines made by Optima Pharma GmbH, a leading German supplier, to ensure protection against contamination for aseptic filling, the company said.
“The filling plant is the culmination of the company’s 15-year history and represents the final piece of the puzzle in our quest to take total control of Ropeg as its development concludes,” PharmaEssentia chairwoman Jan Ching-leou (詹青柳) said in her address.
The company is aiming to manufacture Ropeg entirely in Taiwan, and it has helped the Agricultural Technology Research Institute, its government-supported local drug testing partner, gain certification from the European Medicines Agency (EMA).
Ropeg is nearing the end of the EMA marketing authorization process and the company said it expects to get the green light in Europe before the second quarter next year.
PharmaEssentia has subsidiaries in Beijing, Tokyo and Boston to oversee sales, as opposed to relying on partnerships in foreign markets.
The Taichung plant was certified to produce Ropeg by the Food and Drug Administration (FDA) in 2013 and by the EMA last year, the company said.
The new facility is expected to gain the FDA’s approval by the end of February or March and begin domestic shipments, PharmaEssentia director of biologics manufacturing Cheng Chao-sheng (鄭兆勝) said.
However, the facility would still need to be approved by authorities in the US and Europe, Cheng said, adding that the company is unable to share further details on its regulatory approval timeline.
The facility would provide the company with an ample supply of Ropeg to conduct clinical trials for other indications, Cheng said.
Expanded biologic production capacity would also further a number of other promising candidates in its development pipeline for treating hepatitis B and essential thrombocythemia, as well as breast and gastric cancers, Cheng added.

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
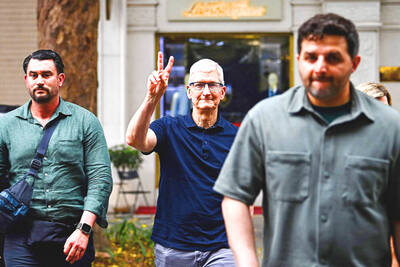
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last