The Industrial Technology Research Institute (ITRI, 工研院) yesterday launched an initiative to speed up the development of the nation’s medical implant industries, beginning with Ingrowth Biotech Co (可成生技), a start-up that focuses on 3D-printing technology.
The government-funded institute has signed letters of intent with partners to establish a precision medicine and clinical translation research and development center.
The center is to serve as a platform to give the nation’s healthcare providers improved software capabilities to help companies tap into the growing market for customized 3D-printed medical implants, ITRI said.
The institute is to work with National Taiwan University Hospital to identify medical needs and diagnostic data, while VtR Inc (法德利科技) is to develop advanced 3D-printing and modeling software similar to that of Dassault Systemes SA, the software development arm of France’s Dassault Group, which played a key role in designing the Mirage 2000 warplane.
Ingrowth, which makes customized 3D-printed orthopedic and dental implants, would be among the first local start-ups to benefit from the platform, ITRI said.
The platform is part of efforts in translational research, which applies findings from basic sciences to produce meaningful health outcomes and new market opportunities as well as cut reliance on imports, the institute said.
About 55 percent of domestic demand for orthopedic implants are met through imports and the trend would only continue to rise as the nation’s population ages, ITRI said.
The initiative would help Taiwan leverage technology to create a localized supply chain for medical implants and help promising projects to progress through clinical studies to reach commercialization, it said.
Ingrowth this year earned ISO 13485 and Good Manufacturing Practices certification for class I, II and III medical devices, and is developing 3D-printed titanium implants for orthopedics and dental applications, the company said.
The company, which is expected post a revenue for the first time next year, has differentiated itself from rivals by leveraging 3D printing to create porous implants designed to support improved biological fixation outcomes after insertion, Ingrowth chairman Ethan Liu (劉永隆) said.
Ingrowth makes implants customized and standardized specifications, and hopes that the partnership would help the company boost precision in setting 3D printing parameters to yield improved production yield rates, Liu said.
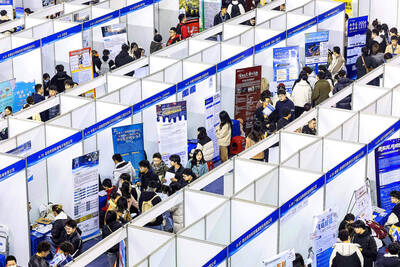
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
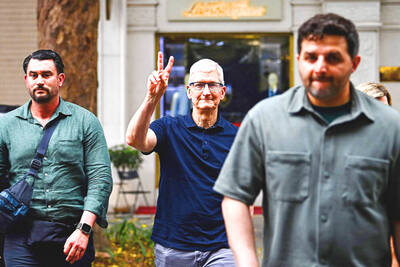
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last

US CONSCULTANT: The US Department of Commerce’s Ursula Burns is a rarely seen US government consultant to be put forward to sit on the board, nominated as an independent director Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday nominated 10 candidates for its new board of directors, including Ursula Burns from the US Department of Commerce. It is rare that TSMC has nominated a US government consultant to sit on its board. Burns was nominated as one of seven independent directors. She is vice chair of the department’s Advisory Council on Supply Chain Competitiveness. Burns is to stand for election at TSMC’s annual shareholders’ meeting on June 4 along with the rest of the candidates. TSMC chairman Mark Liu (劉德音) was not on the list after in December last