The head of the US Food and Drug Administration (FDA) is considering using the agency’s powers to bring more price competition to the market for generic drugs, targeting high-priced products by prioritizing the approval of additional competing treatments.
FDA Commissioner Scott Gottlieb said in an interview on Monday that the agency is looking at how to push applications to the front of the line in cases where there are fewer than three competing generic manufacturers.
The policy would target cases where there are few or no competing versions of drugs, which has led to high prices in some situations.
In one now-infamous case, Turing Pharmaceuticals AG, then led by Martin Shkreli, bought the rights to sell a decades-old anti-infective drug called Daraprim and raised the price from US$13.50 to US$750 per pill.
Valeant Pharmaceuticals International Inc has likewise benefited from older drugs with limited competition, such as two treatments for the rare condition Wilson’s Disease. Valeant bought the treatments and raised their prices by about 30-fold.
Adding generic competitors would mean lower prices, Gottlieb said, adding that the goal is to have three manufacturers of every generic version of a drug.
That is the point at which prices start to fall significantly, he said.
“We know the most significant savings to consumers comes when there are three generics on the market,” Gottlieb said in a telephone interview.
US President Donald Trump has said he wants to address the issue of high US pharmaceutical prices, and drug executives last week said they expected the administration to act soon.
The first generic drug that hits the market will “only slightly lower” the price compared to the brand version, an FDA analysis said.
A second generic competitor approved would slash the price to almost half that of the brand version, while subsequent approvals would drop the cost to 20 percent of the brand version, it added.
The action on generic drugs would be a shift from the agency’s current stance; the FDA prioritizes applications for the first company to apply for a generic version of a brand-name product.
The agency has not typically considered drug costs as a major policy and is barred from taking them into account when deciding whether to approve a new drug.
The move is one of several actions Gottlieb said he plans to take to address drug costs.
The agency is also looking at a plan to eliminate within a year the backlog of 2,640 generic drug applications, Gottlieb said.
Other ideas Gottlieb has referenced include looking into ways to thwart brand-name drugmakers from using programs to restrict distribution of their products to keep generic drug manufacturers from accessing enough product for testing, which he said can require as many as 3,000 pills.
“Getting access to 3,000 tablets can be hard unless the branded companies are going to facilitate the ability of the generic companies to get the drug,” Gottlieb said. “They can’t just go into the market and buy it readily.”
The FDA is also looking into whether it can publish a list of the 180 brand-name drugs that no longer have patent protection and still do not face any generic competition, 150 of which the agency has never received an application from a generic drugmaker to review.
Publishing the list “might create a more compelling business opportunity,” Gottlieb said.
Many of these ideas are to be discussed at a public hearing the FDA plans to hold in the next several months to discuss ways to facilitate generic drug competition, Gottlieb said.
He has also talked about finding ways to speed approvals of generics that copy complex medications, including allergy shots or asthma inhalers such as Mylan NV’s EpiPen or GlaxoSmithKline PLC’s Advair.
The products create an additional complication, because they combine a drug with an administration device.
Gottlieb said he expects the agency to release “a whole series” of documents to guide the industry on the issue, since generic drug manufacturers can run into trouble showing that their versions of the drug and device are equivalent.
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
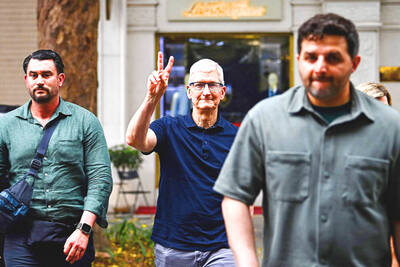
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last