Shares of PharmaEngine Inc (智擎), which develops drugs for cancer and diseases prevalent in Asia, soared by the daily limit yesterday after the company revealed positive results for phase-three clinical trails of a pancreatic cancer treatment.
Shares soared 6.93 percent to close at NT$270 yesterday, outperforming the TAIEX, which rose 0.86 percent.
“The [clinical trial] results indicated that PharmaEngine is likely to receive a drug permit for PEP02, or MM386, by the end of this year in the US and Taiwan, allowing PharmaEngine to book a milestone payment of US$75 million from its US partner Merrimack Pharmaceuticals Inc,” an official of the company, who declined to be named, said over the phone on Thursday.
According to the official, the US$75 million payment will help the pharmaceutical firm swing into the black this year.
Last year, the company’s revenue plunged 79.68 percent to NT$37.4 million (US$1.24 million), from NT$184.03 million in the previous year because of fewer milestone payments, according to the company’s filing with the Taiwan Stock Exchange.
Because of lower revenue, the company reported a loss of NT$117.93 million for last year, while it posted a net profit of NT$48.9 million a year ago, according to the filing.
As for this year, in addition to higher revenue, the company expects to book lower expenses — NT$100 million, down 36.17 percent from NT$156.66 million last year, the official said.
PharmaEngine and Merrimack announced yesterday that the combination of PEP02 and 5-fluorouracil/leucovorin (5-FU/LV) can increase the overall survival of terminally ill patients to 6.1 months instead of the 4.2 months achieved for patients taking only 5-FU/LV. The difference is statistically significant.
Merrimack Pharmaceuticals shares rose 59 percent in New York trading to US$6.99 after the announcement yesterday, outperforming the NASDAQ Composite Index, which was up 0.31 percent.
After the drug is approved in the US, Merrimack will apply for drug permits for MM398 in Europe and Asia, except Taiwan, PharmaEngine said.
PharmaEngine estimated that potential sales of MM386 in Europe and Asia except Taiwan can reach US$500 million a year, PharmaEngine said.
SinoPac Securities Co (永豐金證券) yesterday raised the target price of PharmaEngine to NT$356 from NT$290 because of the results, the bank’s report said.
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see

Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday reported record sales for the first quarter, which analysts attributed to solid demand for emerging technologies. Consolidated revenue totaled NT$592.64 billion (US$18.51 billion) in the January-to-March period, up 16.5 percent from a year earlier, but down 5.26 percent from the previous quarter, TSMC said in a statement. The first-quarter revenue beat analysts’ average projection of NT$579.5 billion, Bloomberg News reported. That performance lends weight to expectations that the world’s most valuable chipmaker would return to solid growth this year after weathering a post-COVID-19-pandemic cratering of smartphone and computer sales. TSMC is budgeting
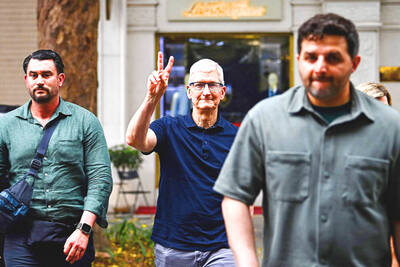
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

US CONSCULTANT: The US Department of Commerce’s Ursula Burns is a rarely seen US government consultant to be put forward to sit on the board, nominated as an independent director Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday nominated 10 candidates for its new board of directors, including Ursula Burns from the US Department of Commerce. It is rare that TSMC has nominated a US government consultant to sit on its board. Burns was nominated as one of seven independent directors. She is vice chair of the department’s Advisory Council on Supply Chain Competitiveness. Burns is to stand for election at TSMC’s annual shareholders’ meeting on June 4 along with the rest of the candidates. TSMC chairman Mark Liu (劉德音) was not on the list after in December last