Japan’s biggest drugs maker, Takeda Pharmaceutical, said yesterday it would fight a huge US$6 billion damages order following a US trial over the safety of its Actos diabetes medicine.
The company said it “respectfully disagrees” with the judgement awarded by a jury in the southern state of Louisiana on Monday, which also ordered the firm’s codefendant, US drugs firm Eli Lilly, to pay US$3 billion in damages.
Investors dumped the Japanese firm’s Tokyo-listed shares, which fell 5.16 percent to ¥4,572 yesterday.
The issue at the trial, which began in February, was whether the drug could be blamed for bladder cancer in a plaintiff who was taking the medicine, and whether the firm knew about those risks, with other US cases still pending.
DISAGREEMENT
“Takeda respectfully disagrees with the verdict and we intend to vigorously challenge this outcome through all available legal means, including possible post-trial motions and an appeal,” said Kenneth Greisman, senior vice president and general counsel for Takeda’s US unit, in a statement.
“We believe the evidence did not support a finding that Actos caused [the plaintiff’s] bladder cancer. We also believe we demonstrated that Takeda acted responsibly with regard to Actos,” Greisman added.
While Takeda rang up about half its US$15 billion sales last fiscal year in Japan, North America and Europe are also major markets and the firm has operations around the world.
Eli Lilly had partnered with Takeda to help market the drug in the US.
Actos — a prescription medication that was launched in 2010 to improve blood sugar control in adults with Type 2 diabetes — had been a promising drug for Takeda, which was recently forced to cancel development of another diabetes treatment due to safety concerns.
SALES PLUNGE
Sales of the medicine — also sold as Pioglitazone — have plunged. They fell 73 percent in the nine months to December last year from the same period a year earlier, according to Takeda’s latest financial statements.
The drug is banned in France.
The US judgement comes about a week after former GlaxoSmithKline executive Christophe Weber was installed as Takeda’s chief operating officer, one of the few foreign-born managers to sit in the top ranks of a Japanese firm.
He is expected to become the company’s president in June.
On Monday, Takeda rival Daiichi Sankyo said it was pulling out of its costly ownership of scandal-hit India drugs maker Ranbaxy, which has been slapped with US import bans linked to its manufacturing practice.
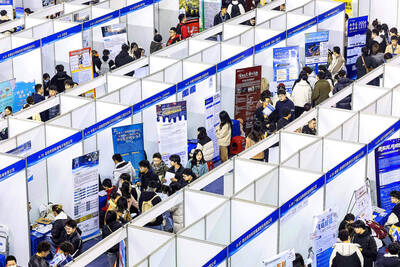
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
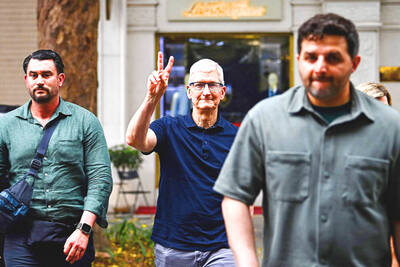
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last