Shares of TWi Pharmaceuticals Inc (安成) fell 9.76 percent after the company said last week that it lost a patent infringement lawsuit against Takeda Pharmaceutical Co of Japan in the US.
The company's shares fell also because some investors took the opportunity to cash in recent gains in the stock.
While the Taiwanese firm said it would appeal, analysts said risks associated with the litigation outcome and other implications would rise accordingly.
On Friday, the company confirmed that the US District Court for the Northern District of California ruled in favor of Takeda’s claims that TWi had infringed on the Japanese firm’s patent No. 7,737,282 concerning an amorphous form of the dexlansopraozle delayed-release capsules, or the generic version of Takeda’s Dexilant.
Dexilant is used to treat heartburn associated with gastroesophageal reflux disease (GERD) and also erosive esophagitis (EE). TWi is seeking to launch a generic version of Dexilant prior to the patents’ expirations, with the latest ending on Sept. 17, 2030.
Citigroup Global Markets estimated that generic Dexilantis, once launched by TWi in 2015, could generate US$25 million revenue to account for 21 percent of the company’s total revenue. The company reported NT$355 million (US$12.1 million) in sales last year.
According to data compiled by researcher IMS Health Inc, the total annual sales of Dexilant in the US were about US$771 million last year.
In April 2011, Takeda initiated the lawsuit against the TWi regarding six patents registered in the US after the latter filed an abbreviated new drug application (ANDA) with a Paragraph IV certification for its generic version of Dexilant in November 2010.
TWi said it had previously prevailed on the other five patents.
“TWi respectfully disagrees with the Court’s decision on the ‘282 patent and intends to appeal the decision,” the company said in a statement on its Web site.
However, First Capital Management Inc (第一金證券投顧) said the company’s move to bring the case to the US Court of Appeals for the Federal Circuit meant it would take six quarters to know the outcome, while Citigroup said risk associated with an unfavorable litigation outcome or a delayed launch of the product has increased, according to their separate client notes yesterday.
TWi shares fell NT$37 to NT$342 on the Emerging Stock Market yesterday. At one point, they lost as much as 12.9 percent, according to the over-the-counter market statistics. So far this year, the shares have surged by nearly 155 percent.
TWi focuses on developing specialty generic prescription drugs for the US market and it has filed nine ANDAs with the US Food and Drug Administration, with plans to launch the drugs in the next three to four years.
The company’s most significant Paragraph IV opportunities are generic versions of Dexilant, Lidoderm (for the relief of pain associated with post-herpetic neuralgia) and Megace ES (used to treat anorexia or wasting of AIDS patients).
TWi has inked US marketing agreements with partners including Teva Pharmaceutical, Par Pharmaceutical and Boca Pharmacal on six drugs on its ANDA pipeline.
First Capital said the company would remain in the red this year, for a net loss of NT$5.5 per share, but forecast a turn around following launches of several generic products over the next few years.
In the first half of the year, TWi reported a net loss per share of NT$3.3, compared with a net loss of NT$2.59 per share in the same period year, according to company data.
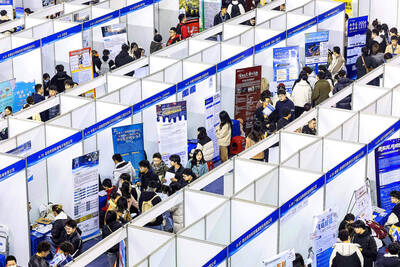
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
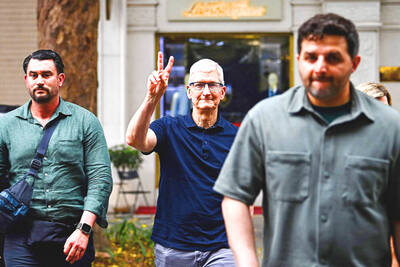
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last

US CONSCULTANT: The US Department of Commerce’s Ursula Burns is a rarely seen US government consultant to be put forward to sit on the board, nominated as an independent director Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday nominated 10 candidates for its new board of directors, including Ursula Burns from the US Department of Commerce. It is rare that TSMC has nominated a US government consultant to sit on its board. Burns was nominated as one of seven independent directors. She is vice chair of the department’s Advisory Council on Supply Chain Competitiveness. Burns is to stand for election at TSMC’s annual shareholders’ meeting on June 4 along with the rest of the candidates. TSMC chairman Mark Liu (劉德音) was not on the list after in December last