The nation’s pharmaceutical industry may face setbacks if certain drugs fail to pass their clinical trials in the next five years, potentially causing investors to pull out their money, Patrick Yang (楊育民), former global head of Roche Pharmaceuticals’ technical operations, said yesterday at a Taipei seminar.
This is because only 10 percent to 13 percent of drugs under development can pass all clinical trials and receive regulatory permits, and even at the phase-three stage of trials, there is still a 30 percent to 40 percent chance for a drug to fail to meet the requirements, Yang said.
“The biochemical industry has improved greatly in the past 10 years as many companies reached certain milestones in developing their drugs, but investors must be aware that a drug could still fail a phase-three clinical trial,” Yang said.
Investors have invested NT$10 billion (US$340.48 million) in local biotechnology and healthcare stocks, but between NT$2 billion to NT$3 billion might be withdrawn by short-term investors, he said.
Investing in biotechnology stocks should be considered a long-term project, he added.
Yang made the statement on the sidelines of Bio Taiwan Commission, a three-day seminar held by the Executive Yuan’s Office of Science and Technology to discuss the strategy of the biomedical industry.
Yang said managers of the Cabinet’s National Development Fund should change their mindset in investing in biotechnological firms.
He said the steering committee operating the fund has expertise in electronics industry, but lacks the knowledge in pharmaceutical industry.
“This committee should not expect to make a profit in under 10 years in the biomedical industry as drug development usually takes a long time,” Yang said.
The Bio Taiwan Commission was yesterday set to discuss whether Taiwan should export its medical services to China and how much the initiative could help local biotechnology industry.
Chang Hong-jen (張鴻仁), chairman and chief executive officer of YFY Biotech Management Co (上騰生技顧問), said he believes it is a great opportunity for Taiwanese biomedical companies to offer services to people in China since there are a lot of new hospitals in that country.
“Our medical management and services have comparative advantage,” Chang said.
However, Soo Whai-jen (蘇懷仁), managing director of Supra Integration and Incubation Center (SIIC), said investing in China might not help the industry to grow.
SIIC is an institute dedicated to promoting the domestic biotechnology industry.
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group
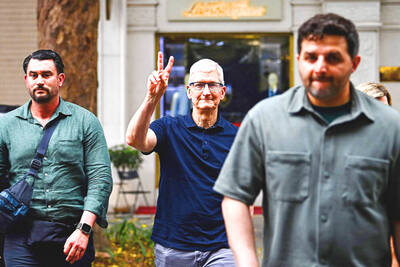
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last