Drug maker PharmaEssentia Corp (藥華醫藥) yesterday said that the US Food and Drug Administration will decide in two weeks whether its new drug P1101 for treating blood disorders can enter phase-three clinical trials directly.
P1101 has passed phase-two trials in Europe, and the Taipei-based pharmaceutical company’s European partner, AOP Orphan Pharmaceuticals AG, has also agreed to share the study data with the company.
That means PharmaEssentia could use the data to apply with the US Food and Drug Administration for clinical trials in the US and thus possibly skip phase-one and phase-two clinical trials there, PharmaEssentia president and chief executive officer Lin Ko-chung (林國鐘) said.
Lin said at a press conference that PharmaEssentia will be responsible for conducting the phase-three study and sell the drug in the US by itself, although it granted AOP Orphan Pharmaceuticals the exclusive rights to sell the drug in Europe.
About 120,000 people in Europe and 200,000 in the US are expected to use the drug, and each will spend US$50,000 a year, Lin said while celebrating the 10th anniversary of the founding of the company, which was started by a group of Taiwanese-American scientists in 2003.
PharmaEssentia plans to launch the drug in both the US and Europe at the beginning of 2016, he added.
Lin said AOP Orphan Pharmaceuticals will give 30 percent of its sales of the drug to PharmaEssentia for royalties and drug manufacturing after it hits the European market.
Lin said the company is also conducting tests to determine whether the drug can treat hepatitis B and C. In Taiwan, hepatitis B and C are the main causes of liver cancer, with between 20 and 25 percent of liver cancer cases triggered by the hepatitis C virus and between 70 and 75 percent by the hepatitis B virus, said Academia Sinica vice president Chen Chien-jen (陳建仁), a former health department minister.
The company has started the phase-two clinical trials of the drug for hepatitis B this quarter and plans to begin the phase-three study of the drug for hepatitis C next quarter, PharmaEssentia said.
“Since liver diseases are most common among Asians, PharmaEssentia will launch the drug for hepatitis B and C in Korea, Thailand and China,” Lin said.
PharmaEssentia will also start the phase-two clinical trials of its drug for treating psoriasis, which will be sold in Taiwan, China and the US this year.
The firm plans to list on the Emerging Stock Market in November or December, and will move to the GRETAI Securities Market or Taiwan Stock Exchange next year.
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group
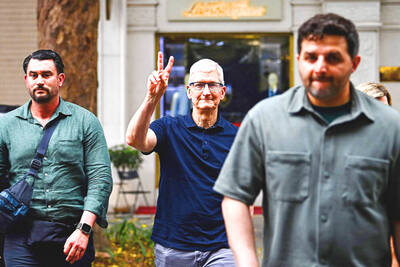
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last