Drug maker PharmaEssentia Corp (藥華醫藥) said yesterday that its new drug for polycythemia vera, a blood disorder of the bone marrow, could be approved in Europe at the end of 2015.
PharmaEssentia licensed the drug to Austrian company AOP Orphan Pharmaceuticals AG in 2009 and will receive milestone payments and royalties from AOP after the drug is approved in Europe and goes on sale.
The drug has completed the second phase of clinical trials in Europe, and PharmaEssentia said it will discuss the European trial data with the US Food and Drug Administration to expedite the approval process to the US.
“We aim to launch the drug in the US and Europe at the beginning of 2016,” company president and chief executive officer Lin Ko-chung (林國鐘) said.
Sales of the drug are expected to reach US$500 million in both the US and Europe, benefiting from higher prices set by both regions as an incentive for drug companies to develop orphan drugs, Lin said.
The company has three patents for the new drug, which will expire in 2028, 2029 and 2030 respectively.
PharmaEssentia built a factory in Central Taiwan Science Park (中部科學園區) last year which has the capacity to produce 1 million units of the drug a year.
In April, the factory won regulatory approval by Taiwan and European medicine agencies for its quality manufacturing process, the company said.
Lin said the company plans to list on the smaller Emerging Stock Market in November or December, and will shift to list on GRETAI Securities Market or Taiwan Stock Exchange next year.
Nutek Corp (閎泰), which manufactures automotive security systems, is the company’s largest shareholder with a 20 percent stake, Lin said, adding that it counts Toyota Motor Corp as one of its major clients.
State-run National Development Fund owns a stake in PharmaEssentia of 12 percent to 13 percent, he said.
Meanwhile, another drug manufacturer SynCore Biotechnology Co (杏國新藥) debuted its shares on the Emerging Stock Market yesterday, seeing its shares rise to NT$114.98 from the initial pubilc offering price of NT$60.
The company's Veregen drug for genital warts has won government's approval and can contribute to its sales starting this quarter, SynCore said.
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group
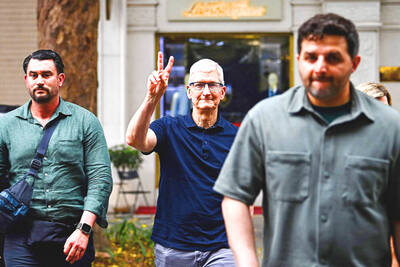
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last