Drug manufacturer Amaran Biotechnology Inc (潤雅生技) yesterday said it is to invest NT$1 billion (US$33.41 million) to build a factory and a research center at Hsinchu Biomedical Science Park (新竹生醫園區) to conduct clinical trials and manufacture drugs developed by OBI Pharma Inc (台灣浩鼎).
The new facilities are set to become operational in the first quarter of next year, the Taipei-based Amaran said.
The factory will produce botulinum toxin type A biosimilar, which is commonly known as Botox and widely used in cosmetic surgery, along with other generic protein drugs, according to the company.
Botox has become very popular for reducing wrinkles and rejuvenating the aging face. It not only has applications in the cosmetics industry but can be used for drugs to treat migraine, squints, severe underarm sweating and dystonia -- sustained muscle contractions that cause twisting and repetitive movements or abnormal postures, according to Amaran.
The company said its new facilities will be the first plant in the nation to be constructed under level 3 biosafety conditions.
The biosafety level 3 is the second-highest on a scale of four, because a high safety level is required for facilities that manufacture Botox in order to limit contamination of the work environment and the surrounding community.
The global market for Botox in 2010 was US$2 billion and it is forecast to grow to US$4.3 billion in 2018, Amaran said, adding that it is the only company in Taiwan that can develop and manufacture that particular type of botulinum toxin.
The research center will be responsible for conducting clinical trials of generic protein drugs developed by Amaran’s partner, OBI Pharma, it said.
Amaran was set up in 2010 as a joint investment between OBI Pharma chairman Michael Chang (張念慈) and Samuel Yin (尹衍樑), the chairman of Ruentex Group (潤泰集團), a business with interests ranging from textiles and construction to retail.
“The reason why Amaran was formed is because we wanted to minimize our risks by letting OBI Pharma focus on the research and development of new drugs, while Amaran is responsible for conducting clinical trials and drug manufacturing,” an OBI Pharma official who declined to be named said by telephone yesterday.
“If our OBI-822/821 drug — which is used to treat breast cancer — can pass the third stage of clinical trials in Taiwan, the new factory will manufacture the drug for us,” she said.
Amaran is also expected to receive investment from Taishin Financial Holding Co (台新金控) chairman Thomas Wu (吳東亮), who attended college with Chang, after Amaran implements a capital enhancement initiative to strengthen its financial structure, which has a paid-in capital of NT$300 million (US$10 million).
Amaran did not give a timeline for the capital enhancement nor the amount that Wu was to invest in the company.
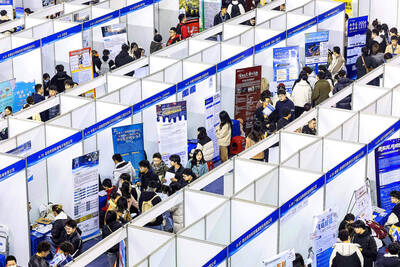
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
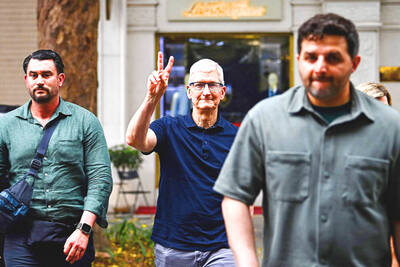
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last

US CONSCULTANT: The US Department of Commerce’s Ursula Burns is a rarely seen US government consultant to be put forward to sit on the board, nominated as an independent director Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday nominated 10 candidates for its new board of directors, including Ursula Burns from the US Department of Commerce. It is rare that TSMC has nominated a US government consultant to sit on its board. Burns was nominated as one of seven independent directors. She is vice chair of the department’s Advisory Council on Supply Chain Competitiveness. Burns is to stand for election at TSMC’s annual shareholders’ meeting on June 4 along with the rest of the candidates. TSMC chairman Mark Liu (劉德音) was not on the list after in December last