Drug maker Taiwan Liposome Co (TLC, 台灣微脂體) yesterday said it has obtained a permit for the production and sale of AmBiL, a generic drug used to cure systematic fungi infection, allowing the sale of the drug in Taiwan by the end of this year providing it receives approval from the Bureau of National Health Insurance for subsidies.
AmBiL would be manufactured and sold in Taiwan by Yungshin Pharmaceutical Industry Co Ltd (永信藥品), TLC said.
Taiwan Liposome said the cheaper AmBiL can compete with AmBisome, the leading drug used for treating the condition, which generated NT$120 million (US$4 million) in local sales last year.
AmBiL is one of the few substitutes for AmBisome that is less toxic, the company added.
Taiwan Liposome will apply for drug permits for AmBiL in the US and Europe, which is expected to take between one and one-and-a-half years, a company official said.
Global sales of AmBisome were US$479 million last year, with most of the money coming from the US and Europe, according to Evaluate Pharma, a provider of pharmaceutical and biotech analysis, said the official, who declined to be named.
Taiwan Liposome is expected to post a profit of NT$38 million this year, compared with losses of NT$187 million last year, after the company last week signed a US$39.5 million deal with US-based SciClone Pharmaceuticals to license its ProFlow drug in China, a Jih Sun Securities Co (日盛證券) forecast said.
The company’s shares rose 5.46 percent to NT$367 yesterday, outperforming the GRETAI’s 0.33 percent decrease.
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
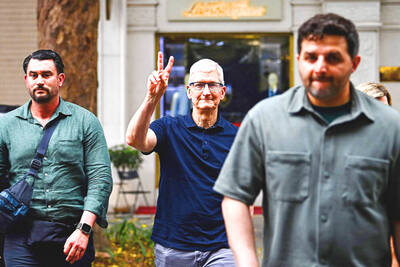
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last

US CONSCULTANT: The US Department of Commerce’s Ursula Burns is a rarely seen US government consultant to be put forward to sit on the board, nominated as an independent director Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday nominated 10 candidates for its new board of directors, including Ursula Burns from the US Department of Commerce. It is rare that TSMC has nominated a US government consultant to sit on its board. Burns was nominated as one of seven independent directors. She is vice chair of the department’s Advisory Council on Supply Chain Competitiveness. Burns is to stand for election at TSMC’s annual shareholders’ meeting on June 4 along with the rest of the candidates. TSMC chairman Mark Liu (劉德音) was not on the list after in December last